The sequence dependence of human nucleotide excision repair efficiencies of benzo[a]pyrene-derived DNA lesions: insights into the structural factors that favor dual incisions
- PMID: 19162041
- PMCID: PMC2717896
- DOI: 10.1016/j.jmb.2008.12.082
The sequence dependence of human nucleotide excision repair efficiencies of benzo[a]pyrene-derived DNA lesions: insights into the structural factors that favor dual incisions
Abstract
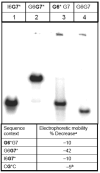
Nucleotide excision repair (NER) is a vital cellular defense system against carcinogen-DNA adducts, which, if not repaired, can initiate cancer development. The structural features of bulky DNA lesions that account for differences in NER efficiencies in mammalian cells are not well understood. In vivo, the predominant DNA adduct derived from metabolically activated benzo[a]pyrene (BP), a prominent environmental carcinogen, is the 10S (+)-trans-anti-[BP]-N(2)-dG adduct (G*), which resides in the B-DNA minor groove 5'-oriented along the modified strand. We have compared the structural distortions in double-stranded DNA, imposed by this adduct, in the different sequence contexts 5'-...CGG*C..., 5'-...CG*GC..., 5'-...CIG*C... (I is 2'-deoxyinosine), and 5'-...CG*C.... On the basis of electrophoretic mobilities, all duplexes manifest moderate bends, except the 5'-...CGG*C...duplex, which exhibits an anomalous, slow mobility attributed to a pronounced flexible kink at the site of the lesion. This kink, resulting from steric hindrance between the 5'-flanking guanine amino group and the BP aromatic rings, both positioned in the minor groove, is abolished in the 5'-...CIG*C...duplex (the 2'-deoxyinosine group, I, lacks this amino group). In contrast, the sequence-isomeric 5'-...CG*GC...duplex exhibits only a moderate bend, but displays a remarkably increased opening rate at the 5'-flanking base pair of G*, indicating a significant destabilization of Watson-Crick hydrogen bonding. The NER dual incision product yields were compared for these different sequences embedded in otherwise identical 135-mer duplexes in cell-free human HeLa extracts. The yields of excision products varied by a factor of as much as approximately 4 in the order 5'-...CG*GC...>5'...CGG*C...>or=5'...CIG*C...>or=5'-...CG*C.... Overall, destabilized Watson-Crick hydrogen bonding, manifested in the 5'-...CG*GC...duplex, elicits the most significant NER response, while the flexible kink displayed in the sequence-isomeric 5'-...CGG*C...duplex represents a less significant signal in this series of substrates. These results demonstrate that the identical lesion can be repaired with markedly variable efficiency in different local sequence contexts that differentially alter the structural features of the DNA duplex around the lesion site.
Figures




Similar articles
-
Differential nucleotide excision repair susceptibility of bulky DNA adducts in different sequence contexts: hierarchies of recognition signals.J Mol Biol. 2009 Jan 9;385(1):30-44. doi: 10.1016/j.jmb.2008.09.087. Epub 2008 Oct 11. J Mol Biol. 2009. PMID: 18948114 Free PMC article.
-
Distant neighbor base sequence context effects in human nucleotide excision repair of a benzo[a]pyrene-derived DNA lesion.J Mol Biol. 2010 Jun 11;399(3):397-409. doi: 10.1016/j.jmb.2010.04.004. Epub 2010 Apr 22. J Mol Biol. 2010. PMID: 20399214 Free PMC article.
-
Solution structure of the (+)-cis-anti-benzo[a]pyrene-dA ([BP]dA) adduct opposite dT in a DNA duplex.Biochemistry. 1999 Aug 17;38(33):10831-42. doi: 10.1021/bi991212f. Biochemistry. 1999. PMID: 10451380
-
DNA adducts from a tumorigenic metabolite of benzo[a]pyrene block human RNA polymerase II elongation in a sequence- and stereochemistry-dependent manner.J Mol Biol. 2002 Aug 2;321(1):29-47. doi: 10.1016/s0022-2836(02)00593-4. J Mol Biol. 2002. PMID: 12139931
-
Repair-Resistant DNA Lesions.Chem Res Toxicol. 2017 Aug 21;30(8):1517-1548. doi: 10.1021/acs.chemrestox.7b00128. Epub 2017 Aug 10. Chem Res Toxicol. 2017. PMID: 28750166 Free PMC article. Review.
Cited by
-
Resistance of bulky DNA lesions to nucleotide excision repair can result from extensive aromatic lesion-base stacking interactions.Nucleic Acids Res. 2011 Nov 1;39(20):8752-64. doi: 10.1093/nar/gkr537. Epub 2011 Jul 15. Nucleic Acids Res. 2011. PMID: 21764772 Free PMC article.
-
Differential nucleotide excision repair susceptibility of bulky DNA adducts in different sequence contexts: hierarchies of recognition signals.J Mol Biol. 2009 Jan 9;385(1):30-44. doi: 10.1016/j.jmb.2008.09.087. Epub 2008 Oct 11. J Mol Biol. 2009. PMID: 18948114 Free PMC article.
-
Synthetic Lesions with a Fluorescein Carbamoyl Group As Analogs of Bulky Lesions Removable by Nucleotide Excision Repair: A Comparative Study on Properties.Acta Naturae. 2024 Jul-Sep;16(3):74-82. doi: 10.32607/actanaturae.27419. Acta Naturae. 2024. PMID: 39555170 Free PMC article.
-
Free energy profiles of base flipping in intercalative polycyclic aromatic hydrocarbon-damaged DNA duplexes: energetic and structural relationships to nucleotide excision repair susceptibility.Chem Res Toxicol. 2013 Jul 15;26(7):1115-25. doi: 10.1021/tx400156a. Epub 2013 Jul 2. Chem Res Toxicol. 2013. PMID: 23758590 Free PMC article.
-
Direct Measurement of 8OG syn-anti Flips in Mutagenic 8OG•A and Long-Range Damage-Dependent Hoogsteen Breathing Dynamics Using 1H CEST NMR.bioRxiv [Preprint]. 2024 Jan 16:2024.01.15.575532. doi: 10.1101/2024.01.15.575532. bioRxiv. 2024. Update in: J Phys Chem B. 2024 May 2;128(17):4087-4096. doi: 10.1021/acs.jpcb.4c00316 PMID: 38293035 Free PMC article. Updated. Preprint.
References
-
- Reardon JT, Sancar A. Nucleotide excision repair. Prog Nucleic Acid Res Mol Biol. 2005;79:183–235. - PubMed
-
- Gillet LC, Scharer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev. 2006;106:253–76. - PubMed
-
- Maillard O, Camenisch U, Blagoev KB, Naegeli H. Versatile protection from mutagenic DNA lesions conferred by bipartite recognition in nucleotide excision repair. Mutation Res. 2008;658:271–286. - PubMed
-
- Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hubscher U, Egly JM, Wood RD. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

