Blocking interhost transmission of influenza virus by vaccination in the guinea pig model
- PMID: 19153237
- PMCID: PMC2655561
- DOI: 10.1128/JVI.02424-08
Blocking interhost transmission of influenza virus by vaccination in the guinea pig model
Abstract
Interventions aimed at preventing viral spread have the potential to effectively control influenza virus in all age groups, thereby reducing the burden of influenza illness. For this reason, we have examined the efficacy of vaccination in blocking the transmission of influenza viruses between guinea pigs. Three modes of immunization were compared: (i) natural infection; (ii) intramuscular administration of whole, inactivated influenza virus in 2 doses; and (iii) intranasal inoculation with live attenuated influenza virus in 2 doses. The ability of each immunization method to block the spread of a homologous (A/Panama/2007/99) H3N2 subtype and a heterologous (A/Wisconsin/67/05) H3N2 subtype influenza virus was tested. We found that previous infection through a natural route provided sterilizing immunity against both homologous and heterologous challenges; thus, no transmission to or from previously infected animals was observed. Vaccination with an inactivated influenza virus vaccine, in contrast, did not prevent guinea pigs from becoming infected upon challenge with either virus. Thus, both intranasal inoculation and exposure to an acutely infected guinea pig led to the infection of vaccinated animals. Vaccination with inactivated virus did, however, reduce viral load upon challenge and decrease the number of secondary transmission events from vaccinated animals to naïve cage mates. Vaccination with a live attenuated virus was found to be more efficacious than vaccination with inactivated virus, resulting in sterilizing immunity against homologous challenge and full protection against the transmission of the homologous and heterologous viruses to naïve contacts. In conclusion, we have shown that the guinea pig model can be used to test influenza virus vaccines and that the efficiency of transmission is a valuable readout when vaccine efficacy is evaluated.
Figures
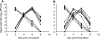


Similar articles
-
Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011-2012 H3N2v.J Virol. 2013 Sep;87(17):9895-903. doi: 10.1128/JVI.01038-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824815 Free PMC article.
-
Vaccines That Reduce Viral Shedding Do Not Prevent Transmission of H1N1 Pandemic 2009 Swine Influenza A Virus Infection to Unvaccinated Pigs.J Virol. 2021 Jan 28;95(4):e01787-20. doi: 10.1128/JVI.01787-20. Print 2021 Jan 28. J Virol. 2021. PMID: 33268518 Free PMC article.
-
Poly I:C adjuvanted inactivated swine influenza vaccine induces heterologous protective immunity in pigs.Vaccine. 2015 Jan 15;33(4):542-8. doi: 10.1016/j.vaccine.2014.11.034. Epub 2014 Nov 28. Vaccine. 2015. PMID: 25437101 Free PMC article.
-
Efficacy of intranasal administration of a truncated NS1 modified live influenza virus vaccine in swine.Vaccine. 2007 Nov 19;25(47):7999-8009. doi: 10.1016/j.vaccine.2007.09.019. Epub 2007 Sep 29. Vaccine. 2007. PMID: 17933442 Free PMC article. Clinical Trial.
-
Efficacy of Heterologous Prime-Boost Vaccination with H3N2 Influenza Viruses in Pre-Immune Individuals: Studies in the Pig Model.Viruses. 2020 Sep 1;12(9):968. doi: 10.3390/v12090968. Viruses. 2020. PMID: 32882956 Free PMC article.
Cited by
-
Predicting 'airborne' influenza viruses: (trans-) mission impossible?Curr Opin Virol. 2011 Dec;1(6):635-42. doi: 10.1016/j.coviro.2011.07.003. Epub 2011 Sep 3. Curr Opin Virol. 2011. PMID: 22440921 Free PMC article. Review.
-
Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity.PLoS One. 2012;7(12):e51559. doi: 10.1371/journal.pone.0051559. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236516 Free PMC article.
-
New class of monoclonal antibodies against severe influenza: prophylactic and therapeutic efficacy in ferrets.PLoS One. 2010 Feb 8;5(2):e9106. doi: 10.1371/journal.pone.0009106. PLoS One. 2010. PMID: 20161706 Free PMC article.
-
Influenza B: Prospects for the Development of Cross-Protective Vaccines.Viruses. 2022 Jun 17;14(6):1323. doi: 10.3390/v14061323. Viruses. 2022. PMID: 35746794 Free PMC article. Review.
-
Animal Models for Influenza Virus Pathogenesis and Transmission.Viruses. 2010;2(8):1530-1563. doi: 10.3390/v20801530. Viruses. 2010. PMID: 21442033 Free PMC article.
References
-
- Belshe, R. B. 2004. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 103177-185. - PubMed
-
- Belshe, R. B. 2007. Translational research on vaccines: influenza as an example. Clin. Pharmacol. Ther. 82745-749. - PubMed
-
- Belshe, R. B., K. M. Edwards, T. Vesikari, S. V. Black, R. E. Walker, M. Hultquist, G. Kemble, and E. M. Connor. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 356685-696. - PubMed
-
- Belshe, R. B., W. C. Gruber, P. M. Mendelman, I. Cho, K. Reisinger, S. L. Block, J. Wittes, D. Iacuzio, P. Piedra, J. Treanor, J. King, K. Kotloff, D. I. Bernstein, F. G. Hayden, K. Zangwill, L. Yan, and M. Wolff. 2000. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J. Pediatr. 136168-175. - PubMed
-
- Beyer, W. E. P., A. M. Palache, J. C. de Jong, and A. D. M. E. Osterhaus. 2002. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy: a meta-analysis. Vaccine 201340-1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

