Carbohydrate-dependent defense mechanisms against Helicobacter pylori infection
- PMID: 19149511
- PMCID: PMC2666621
- DOI: 10.2174/138920009787048428
Carbohydrate-dependent defense mechanisms against Helicobacter pylori infection
Abstract
Helicobacter pylori is a Gram-negative bacterium that infects over 50% of the world's population. This organism causes various gastric diseases such as chronic gastritis, peptic ulcer, and gastric cancer. H. pylori possesses lipopolysaccharide, which shares structural similarity to Lewis blood group antigens in gastric mucosa. Such antigenic mimicry could result in immune tolerance against antigens of this pathogen. On the other hand, H. pylori colonize gastric mucosa by utilizing adhesins, which bind Lewis blood group antigen-related carbohydrates expressed on gastric epithelial cells. In chronic gastritis, lymphocytes infiltrate the lamina propria, and such infiltration is facilitated by 6-sulfo sialyl Lewis X-capped O-glycans, peripheral lymph node addressin (PNAd), on high endothelial venule (HEV)-like vessels. The number of HEV-like vessels increases as chronic inflammation progresses. Furthermore, PNAd formed on HEV-like vessels disappear once H. pylori is eradicated. These results indicate that PNAd plays an important role in H. pylori-associated inflammation. H. pylori barely colonizes gland mucous cell-derived mucin where alpha1,4-GlcNAc-capped O-glycans exist. In vitro experiments show that alpha1,4-GlcNAc-capped O-glycans function as a natural antibiotic to inhibit H. pylori growth. We recently identified cholesterol alpha-glucosyltransferase (CHLalphaGcT) using an expression cloning strategy and showed that this enzyme is specifically inhibited by mucin-type O-glycans like those present in deeper portions of the gastric mucosa. These findings show that a battery of carbohydrates expressed in the stomach is closely associated with pathogenesis and also prevention of H. pylori-related diseases.
Figures





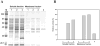
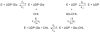
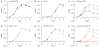
Similar articles
-
Roles of gastric mucin-type O-glycans in the pathogenesis of Helicobacter pylori infection.Glycobiology. 2009 May;19(5):453-61. doi: 10.1093/glycob/cwp004. Epub 2009 Jan 15. Glycobiology. 2009. PMID: 19150806 Free PMC article. Review.
-
Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori.Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17807-12. doi: 10.1073/pnas.0407503101. Epub 2004 Dec 9. Proc Natl Acad Sci U S A. 2004. PMID: 15591109 Free PMC article.
-
Role of sulfated O-glycans expressed by high endothelial venule-like vessels in pathogenesis of chronic inflammatory gastrointestinal diseases.Biol Pharm Bull. 2009 May;32(5):774-9. doi: 10.1248/bpb.32.774. Biol Pharm Bull. 2009. PMID: 19420741 Free PMC article. Review.
-
Human trefoil factor 2 is a lectin that binds α-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori.J Biol Chem. 2014 Oct 3;289(40):27363-75. doi: 10.1074/jbc.M114.597757. Epub 2014 Aug 14. J Biol Chem. 2014. PMID: 25124036 Free PMC article.
-
Prominent expression of sialyl Lewis X-capped core 2-branched O-glycans on high endothelial venule-like vessels in gastric MALT lymphoma.J Pathol. 2011 May;224(1):67-77. doi: 10.1002/path.2851. Epub 2011 Mar 22. J Pathol. 2011. PMID: 21432854 Free PMC article.
Cited by
-
Chemoenzymatic synthesis of cholesteryl-6-O-tetradecanoyl-α-D-glucopyranoside: a product of host cholesterol efflux promoted by Helicobacter pylori.Chem Commun (Camb). 2012 Sep 18;48(72):9083-5. doi: 10.1039/c2cc33948j. Epub 2012 Aug 2. Chem Commun (Camb). 2012. PMID: 22854787 Free PMC article.
-
Helicobacter pylori and skin autoimmune diseases.World J Gastroenterol. 2014 Feb 14;20(6):1510-6. doi: 10.3748/wjg.v20.i6.1510. World J Gastroenterol. 2014. PMID: 24587626 Free PMC article. Review.
-
Glycoside hydrolase family 89 alpha-N-acetylglucosaminidase from Clostridium perfringens specifically acts on GlcNAc alpha1,4Gal beta1R at the non-reducing terminus of O-glycans in gastric mucin.J Biol Chem. 2011 Feb 25;286(8):6479-89. doi: 10.1074/jbc.M110.206722. Epub 2010 Dec 21. J Biol Chem. 2011. PMID: 21177247 Free PMC article.
-
Tandem glycosyl iodide glycosylation and regioselective enzymatic acylation affords 6-O-tetradecanoyl-α-d-cholesterylglycosides.J Org Chem. 2014 Sep 5;79(17):8447-52. doi: 10.1021/jo501371h. Epub 2014 Aug 13. J Org Chem. 2014. PMID: 25093454 Free PMC article.
-
Chemoenzymatic Synthesis of Tri-antennary N-Glycans Terminating in Sialyl-Lewisx Reveals the Importance of Glycan Complexity for Influenza A Virus Receptor Binding.Chemistry. 2024 Jun 6;30(32):e202401108. doi: 10.1002/chem.202401108. Epub 2024 May 8. Chemistry. 2024. PMID: 38567703
References
-
- Doenges JL. Spirochaetes in gastric glands of macacus rhesus and humans without definite history of related disease. Proc Soc Exp Biol Med. 1938;38:536–538.
-
- Freedburg AS, Barron LE. The presence of spirochaetes in human gastric mucosa. Am J Dig Dis. 1940;7:443–445.
-
- Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;321(8336):1273–1275. - PubMed
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;323(8390):1311–1315. - PubMed
-
- Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch’s postulates for pyloric Campylobacter Med. J Aust. 1985;142(8):436–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

