Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells
- PMID: 19147228
- PMCID: PMC3047414
- DOI: 10.1016/j.leukres.2008.11.024
Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells
Abstract
An emerging treatment option for chronic lymphocytic leukemia (CLL) is to make cytotoxic immune cells express a chimeric antigen receptor (CAR) that recognizes specific surface molecules on CLL cells. Here an mRNA coding for an anti-CD19 CAR was transfected into the NK-92 cell line by electroporation. In contrast to cDNA, mRNA resulted in high transfection efficiency (47.2 +/- 8% versus <5% for cDNA) with minimal effect on cell viability. NK-92 cells expressing anti-CD19 CAR killed previously resistant CD19+ B-ALL cell lines, as well as primary CLL cells and therefore may present a safe, cell-based, targeted treatment for patients with CLL.
Conflict of interest statement
H. Klingemann is co-founder and shareholder in ZelleRx Corp.
Figures


 ), either in the form of DNA or mRNA, or without any nucleic acid (□, control).
), either in the form of DNA or mRNA, or without any nucleic acid (□, control).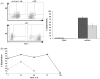
 ) proteins at 24 h post-electroporation in NK-92 cells electroporated with DNA or mRNA, shown in percentage of the whole cell population. (B) Expression of GFP (●) or αCD19-CAR (
) proteins at 24 h post-electroporation in NK-92 cells electroporated with DNA or mRNA, shown in percentage of the whole cell population. (B) Expression of GFP (●) or αCD19-CAR ( ) proteins over time in hours in NK-92 cells electroporated with mRNA.
) proteins over time in hours in NK-92 cells electroporated with mRNA.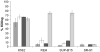
 ), and electroporated without nucleic acid (□), with GFP mRNA (■), or with αCD19-CAR mRNA (
), and electroporated without nucleic acid (□), with GFP mRNA (■), or with αCD19-CAR mRNA ( ), against cell lines K562, REH, SUP-B15, and SR-91. The data correspond to an effector to target ratio of 5:1.
), against cell lines K562, REH, SUP-B15, and SR-91. The data correspond to an effector to target ratio of 5:1.
 ), against five primary CLL MNC samples. The data are for an effector to target ratio of 5:1.
), against five primary CLL MNC samples. The data are for an effector to target ratio of 5:1.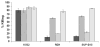
 ).
).Similar articles
-
Large volume flow electroporation of mRNA: clinical scale process.Methods Mol Biol. 2013;969:127-38. doi: 10.1007/978-1-62703-260-5_9. Methods Mol Biol. 2013. PMID: 23296932 Review.
-
Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens.Leuk Lymphoma. 2012 May;53(5):958-65. doi: 10.3109/10428194.2011.634048. Epub 2011 Dec 6. Leuk Lymphoma. 2012. PMID: 22023526 Free PMC article.
-
Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors.N Engl J Med. 2020 Feb 6;382(6):545-553. doi: 10.1056/NEJMoa1910607. N Engl J Med. 2020. PMID: 32023374 Free PMC article. Clinical Trial.
-
Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma.Cytotherapy. 2017 Feb;19(2):235-249. doi: 10.1016/j.jcyt.2016.10.009. Epub 2016 Nov 22. Cytotherapy. 2017. PMID: 27887866
-
NK-92 cell, another ideal carrier for chimeric antigen receptor.Immunotherapy. 2017 Aug;9(9):753-765. doi: 10.2217/imt-2017-0022. Epub 2017 Aug 3. Immunotherapy. 2017. PMID: 28771105 Review.
Cited by
-
Advantages and applications of CAR-expressing natural killer cells.Front Pharmacol. 2015 Feb 12;6:21. doi: 10.3389/fphar.2015.00021. eCollection 2015. Front Pharmacol. 2015. PMID: 25729364 Free PMC article. Review.
-
The Advent of CAR T-Cell Therapy for Lymphoproliferative Neoplasms: Integrating Research Into Clinical Practice.Front Immunol. 2020 May 12;11:888. doi: 10.3389/fimmu.2020.00888. eCollection 2020. Front Immunol. 2020. PMID: 32477359 Free PMC article. Review.
-
Synergistic Effects of Cabozantinib and EGFR-Specific CAR-NK-92 Cells in Renal Cell Carcinoma.J Immunol Res. 2017;2017:6915912. doi: 10.1155/2017/6915912. Epub 2017 Dec 20. J Immunol Res. 2017. PMID: 29423418 Free PMC article.
-
Aptamer-Engineered Natural Killer Cells for Cell-Specific Adaptive Immunotherapy.Small. 2019 May;15(22):e1900903. doi: 10.1002/smll.201900903. Epub 2019 Apr 26. Small. 2019. PMID: 31026116 Free PMC article.
-
Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor.Mol Ther. 2015 Feb;23(2):330-8. doi: 10.1038/mt.2014.219. Epub 2014 Nov 6. Mol Ther. 2015. PMID: 25373520 Free PMC article.
References
-
- Shanafelt TD, Byrd JC, Call TG, Zent CS, Kay NE. Narrative review: initial management of newly diagnosed, early-stage chronic lymphocytic leukemia. Ann Intern Med. 2006;145(6):435–47. - PubMed
-
- Rozman C, Bosch F, Montserrat E. Chronic lymphocytic leukemia: a changing natural history? Leukemia. 1997;11(6):775–8. - PubMed
-
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. - PubMed
-
- Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100(6):1935–47. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

