Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis
- PMID: 19147016
- PMCID: PMC2631421
- DOI: 10.1016/j.ijrobp.2008.09.036
Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis
Abstract
Purpose: Radiotherapy for head-and-neck cancer causes adverse secondary side effects in the salivary glands and results in diminished quality of life for the patient. A previous in vivo study in parotid salivary glands demonstrated that targeted head-and-neck irradiation resulted in marked increases in phosphorylated p53 (serine(18)) and apoptosis, which was suppressed in transgenic mice expressing a constitutively active mutant of Akt1 (myr-Akt1).
Methods and materials: Transgenic and knockout mouse models were exposed to irradiation, and p53-mediated transcription, apoptosis, and salivary gland dysfunction were analyzed.
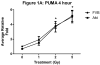
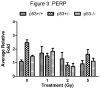
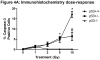
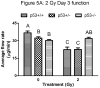
Results: The proapoptotic p53 target genes PUMA and Bax were induced in parotid salivary glands of mice at early time points after therapeutic radiation. This dose-dependent induction requires expression of p53 because no radiation-induced expression of PUMA and Bax was observed in p53-/- mice. Radiation also induced apoptosis in the parotid gland in a dose-dependent manner, which was p53 dependent. Furthermore, expression of p53 was required for the acute and chronic loss of salivary function after irradiation. In contrast, apoptosis was not induced in p53-/- mice, and their salivary function was preserved after radiation exposure.
Conclusions: Apoptosis in the salivary glands after therapeutic head-and-neck irradiation is mediated by p53 and corresponds to salivary gland dysfunction in vivo.
Conflict of interest statement
Figures








Similar articles
-
MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells.Mol Cell Biol. 2006 Dec;26(23):8840-56. doi: 10.1128/MCB.01846-05. Epub 2006 Sep 18. Mol Cell Biol. 2006. PMID: 16982679 Free PMC article.
-
IGF1 activates cell cycle arrest following irradiation by reducing binding of ΔNp63 to the p21 promoter.Cell Death Dis. 2010;1(6):e50. doi: 10.1038/cddis.2010.28. Cell Death Dis. 2010. PMID: 21480565 Free PMC article.
-
The proapoptotic BH3-only, Bcl-2 family member, Puma is critical for acute ethanol-induced neuronal apoptosis.J Neuropathol Exp Neurol. 2009 Jul;68(7):747-56. doi: 10.1097/NEN.0b013e3181a9d524. J Neuropathol Exp Neurol. 2009. PMID: 19535997 Free PMC article.
-
On the mechanism of salivary gland radiosensitivity.Int J Radiat Oncol Biol Phys. 2005 Jul 15;62(4):1187-94. doi: 10.1016/j.ijrobp.2004.12.051. Int J Radiat Oncol Biol Phys. 2005. PMID: 15990024 Review.
-
Sensitivity of salivary glands to radiation: from animal models to therapies.J Dent Res. 2009 Oct;88(10):894-903. doi: 10.1177/0022034509343143. J Dent Res. 2009. PMID: 19783796 Free PMC article. Review.
Cited by
-
Long-term Pilocarpine Treatment Improves Salivary Flow in Irradiated Mice.Acta Histochem Cytochem. 2019 Jun 25;52(3):45-58. doi: 10.1267/ahc.19006. Epub 2019 Jun 19. Acta Histochem Cytochem. 2019. PMID: 31341340 Free PMC article.
-
Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress.Antioxidants (Basel). 2021 Oct 22;10(11):1666. doi: 10.3390/antiox10111666. Antioxidants (Basel). 2021. PMID: 34829539 Free PMC article. Review.
-
Label-retaining cells in the adult murine salivary glands possess characteristics of adult progenitor cells.PLoS One. 2014 Sep 19;9(9):e107893. doi: 10.1371/journal.pone.0107893. eCollection 2014. PLoS One. 2014. PMID: 25238060 Free PMC article.
-
Short-term and bystander effects of radiation on murine submandibular glands.Dis Model Mech. 2022 Nov 1;15(11):dmm049570. doi: 10.1242/dmm.049570. Epub 2022 Nov 10. Dis Model Mech. 2022. PMID: 36263624 Free PMC article.
-
Prevention of radiation-induced salivary gland dysfunction utilizing a CDK inhibitor in a mouse model.PLoS One. 2012;7(12):e51363. doi: 10.1371/journal.pone.0051363. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236487 Free PMC article.
References
-
- Nagler RM. Effects of head and neck radiotherapy on major salivary glands--animal studies and human implications. In Vivo. 2003;17(4):369–375. - PubMed
-
- Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):577–587. - PubMed
-
- Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the PKCdelta null mouse in vivo. J Biol Chem. 2006;281(14):9728–9737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

