Acute lymphoblastic leukemia-associated JAK1 mutants activate the Janus kinase/STAT pathway via interleukin-9 receptor alpha homodimers
- PMID: 19139102
- PMCID: PMC2652315
- DOI: 10.1074/jbc.M807531200
Acute lymphoblastic leukemia-associated JAK1 mutants activate the Janus kinase/STAT pathway via interleukin-9 receptor alpha homodimers
Abstract
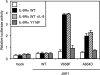
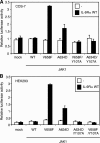
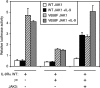
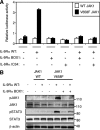
Activating mutations in JAK1 have been reported in acute lymphoblastic leukemias, but little is known about the mechanisms involved in their constitutive activation. Here, we studied the ability of JAK1 V658F and A634D to activate the Janus kinase (JAK)/STAT pathway upon ectopic expression in HEK293 cells alone or together with the other components of the interleukin-9 receptor complex (IL-9Ralpha, gammac, and JAK3). Expression of JAK1 mutants alone failed to trigger STAT activation, but co-expression of the IL-9Ralpha chain promoted JAK1 mutant phosphorylation and STAT activation. Mutation of the FERM domain of JAK1, which is critical for cytokine receptor association, or of the single tyrosine of IL-9Ralpha involved in STAT recruitment abolished this activity, indicating that JAK1 mutants need to associate with a functional IL-9Ralpha to activate STAT factors. Several lines of evidence indicated that IL-9Ralpha homodimerization was involved in this process. IL-9Ralpha variants with mutations of the JAK-interacting BOX1 region not only failed to promote JAK1 activation but also acted as dominant negative forms reverting the effect of wild-type IL-9Ralpha. Coimmunoprecipitation experiments also showed the formation of IL-9Ralpha homodimers. Interestingly, STAT activation was partially inhibited by expression of gammac, suggesting that overlapping residues are involved in IL-9Ralpha homodimerization and IL-9Ralpha/gammac heterodimerization. Co-expression of wild-type JAK3 partially reverted the inhibition by gammac, indicating that JAK3 cooperates with JAK1 mutants within the IL-9 receptor complex. Similar results were observed with IL-2Rbeta. Taken together, our results show that IL-9Ralpha and IL-2Rbeta homodimers efficiently mediate constitutive activation of ALL-associated JAK1 mutants.
Figures


Similar articles
-
Ligand-independent homomeric and heteromeric complexes between interleukin-2 or -9 receptor subunits and the gamma chain.J Biol Chem. 2008 Nov 28;283(48):33569-77. doi: 10.1074/jbc.M803125200. Epub 2008 Oct 1. J Biol Chem. 2008. PMID: 18829468 Free PMC article.
-
Distinct Acute Lymphoblastic Leukemia (ALL)-associated Janus Kinase 3 (JAK3) Mutants Exhibit Different Cytokine-Receptor Requirements and JAK Inhibitor Specificities.J Biol Chem. 2015 Nov 27;290(48):29022-34. doi: 10.1074/jbc.M115.670224. Epub 2015 Oct 7. J Biol Chem. 2015. PMID: 26446793 Free PMC article.
-
Receptor expression is essential for proliferation induced by dimerized Jak kinases.Biochem Biophys Res Commun. 2008 Jun 13;370(4):557-60. doi: 10.1016/j.bbrc.2008.03.095. Epub 2008 Mar 31. Biochem Biophys Res Commun. 2008. PMID: 18381062
-
JAK/STAT signaling in hematological malignancies.Oncogene. 2013 May 23;32(21):2601-13. doi: 10.1038/onc.2012.347. Epub 2012 Aug 6. Oncogene. 2013. PMID: 22869151 Review.
-
JAK/STAT pathway directed therapy of T-cell leukemia/lymphoma: Inspired by functional and structural genomics.Mol Cell Endocrinol. 2017 Aug 15;451:66-70. doi: 10.1016/j.mce.2017.02.019. Epub 2017 Feb 15. Mol Cell Endocrinol. 2017. PMID: 28214593 Free PMC article. Review.
Cited by
-
Overexpression of IL-9 induced by STAT6 activation promotes the pathogenesis of chronic lymphocytic leukemia.Int J Clin Exp Pathol. 2014 Apr 15;7(5):2319-23. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24966942 Free PMC article.
-
Cytokine receptor signaling is required for the survival of ALK- anaplastic large cell lymphoma, even in the presence of JAK1/STAT3 mutations.Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):3975-3980. doi: 10.1073/pnas.1700682114. Epub 2017 Mar 29. Proc Natl Acad Sci U S A. 2017. PMID: 28356514 Free PMC article.
-
Association between low expression levels of interleukin-9 and colon cancer progression.Exp Ther Med. 2015 Sep;10(3):942-946. doi: 10.3892/etm.2015.2588. Epub 2015 Jun 23. Exp Ther Med. 2015. PMID: 26622419 Free PMC article.
-
Evaluation of Interleukin-9 Expression as a Potential Therapeutic Target in Chronic Lymphocytic Leukemia in a Cohort of Egyptian Patients.Indian J Hematol Blood Transfus. 2017 Dec;33(4):477-482. doi: 10.1007/s12288-017-0804-1. Epub 2017 Mar 17. Indian J Hematol Blood Transfus. 2017. PMID: 29075057 Free PMC article.
-
Identification of a novel functional JAK1 S646P mutation in acute lymphoblastic leukemia.Oncotarget. 2017 May 23;8(21):34687-34697. doi: 10.18632/oncotarget.16670. Oncotarget. 2017. PMID: 28410228 Free PMC article.
References
-
- Schindler, C., Levy, D. E., and Decker, T. (2007) J. Biol. Chem. 282 20059–20063 - PubMed
-
- James, C., Ugo, V., Le Couedic, J. P., Staerk, J., Delhommeau, F., Lacout, C., Garcon, L., Raslova, H., Berger, R., Bennaceur-Griscelli, A., Villeval, J. L., Constantinescu, S. N., Casadevall, N., and Vainchenker, W. (2005) Nature 434 1144–1148 - PubMed
-
- Kralovics, R., Passamonti, F., Buser, A. S., Teo, S. S., Tiedt, R., Passweg, J. R., Tichelli, A., Cazzola, M., and Skoda, R. C. (2005) N. Engl. J. Med. 352 1779–1790 - PubMed
-
- Levine, R. L., Wadleigh, M., Cools, J., Ebert, B. L., Wernig, G., Huntly, B. J., Boggon, T. J., Wlodarska, I., Clark, J. J., Moore, S., Adelsperger, J., Koo, S., Lee, J. C., Gabriel, S., Mercher, T., D'Andrea, A., Frohling, S., Dohner, K., Marynen, P., Vandenberghe, P., Mesa, R. A., Tefferi, A., Griffin, J. D., Eck, M. J., Sellers, W. R., Meyerson, M., Golub, T. R., Lee, S. J., and Gilliland, D. G. (2005) Cancer Cell 7 387–397 - PubMed
-
- Baxter, E. J., Scott, L. M., Campbell, P. J., East, C., Fourouclas, N., Swanton, S., Vassiliou, G. S., Bench, A. J., Boyd, E. M., Curtin, N., Scott, M. A., Erber, W. N., and Green, A. R. (2005) Lancet 365 1054–1061 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

