Rapid determination of hydrogen positions and protonation states of diisopropyl fluorophosphatase by joint neutron and X-ray diffraction refinement
- PMID: 19136630
- PMCID: PMC2630072
- DOI: 10.1073/pnas.0807842106
Rapid determination of hydrogen positions and protonation states of diisopropyl fluorophosphatase by joint neutron and X-ray diffraction refinement
Abstract
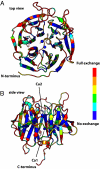
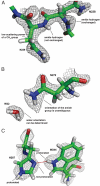
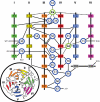
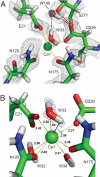
Hydrogen atoms constitute about half of all atoms in proteins and play a critical role in enzyme mechanisms and macromolecular and solvent structure. Hydrogen atom positions can readily be determined by neutron diffraction, and as such, neutron diffraction is an invaluable tool for elucidating molecular mechanisms. Joint refinement of neutron and X-ray diffraction data can lead to improved models compared with the use of neutron data alone and has now been incorporated into modern, maximum-likelihood based crystallographic refinement programs like CNS. Joint refinement has been applied to neutron and X-ray diffraction data collected on crystals of diisopropyl fluorophosphatase (DFPase), a calcium-dependent phosphotriesterase capable of detoxifying organophosphorus nerve agents. Neutron omit maps reveal a number of important features pertaining to the mechanism of DFPase. Solvent molecule W33, coordinating the catalytic calcium, is a water molecule in a strained coordination environment, and not a hydroxide. The smallest Ca-O-H angle is 53 degrees, well beyond the smallest angles previously observed. Residue Asp-229, is deprotonated, supporting a mechanism involving nucleophilic attack by Asp-229, and excluding water activation by the catalytic calcium. The extended network of hydrogen bonding interactions in the central water filled tunnel of DFPase is revealed, showing that internal solvent molecules form an important, integrated part of the overall structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
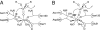
Similar articles
-
Neutron structure and mechanistic studies of diisopropyl fluorophosphatase (DFPase).Acta Crystallogr D Biol Crystallogr. 2010 Nov;66(Pt 11):1131-8. doi: 10.1107/S0907444910034013. Epub 2010 Oct 20. Acta Crystallogr D Biol Crystallogr. 2010. PMID: 21041927 Free PMC article.
-
Hydrogen atoms in protein structures: high-resolution X-ray diffraction structure of the DFPase.BMC Res Notes. 2013 Aug 2;6:308. doi: 10.1186/1756-0500-6-308. BMC Res Notes. 2013. PMID: 23915572 Free PMC article.
-
Preliminary time-of-flight neutron diffraction study on diisopropyl fluorophosphatase (DFPase) from Loligo vulgaris.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 Jan 1;63(Pt 1):42-5. doi: 10.1107/S1744309106052924. Epub 2006 Dec 22. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007. PMID: 17183172 Free PMC article.
-
Neutron protein crystallography: A complementary tool for locating hydrogens in proteins.Arch Biochem Biophys. 2016 Jul 15;602:48-60. doi: 10.1016/j.abb.2015.11.033. Epub 2015 Nov 22. Arch Biochem Biophys. 2016. PMID: 26592456 Review.
-
Large crystal growth by thermal control allows combined X-ray and neutron crystallographic studies to elucidate the protonation states in Aspergillus flavus urate oxidase.J R Soc Interface. 2009 Oct 6;6 Suppl 5(Suppl 5):S599-610. doi: 10.1098/rsif.2009.0162.focus. Epub 2009 Jul 8. J R Soc Interface. 2009. PMID: 19586953 Free PMC article. Review.
Cited by
-
Advancements in bioscavenger mediated detoxification of organophosphorus poisoning.Toxicol Res (Camb). 2024 Jun 10;13(3):tfae089. doi: 10.1093/toxres/tfae089. eCollection 2024 Jun. Toxicol Res (Camb). 2024. PMID: 38863796 Review.
-
Warfare Nerve Agents and Paraoxonase-1 as a Potential Prophylactic Therapy against Intoxication.Protein Pept Lett. 2024;31(5):345-355. doi: 10.2174/0109298665284293240409045359. Protein Pept Lett. 2024. PMID: 38706353 Review.
-
Probing the Suitability of Different Ca2+ Parameters for Long Simulations of Diisopropyl Fluorophosphatase.Molecules. 2021 Sep 26;26(19):5839. doi: 10.3390/molecules26195839. Molecules. 2021. PMID: 34641383 Free PMC article.
-
Theoretical Studies on Catalysis Mechanisms of Serum Paraoxonase 1 and Phosphotriesterase Diisopropyl Fluorophosphatase Suggest the Alteration of Substrate Preference from Paraoxonase to DFP.Molecules. 2018 Jul 7;23(7):1660. doi: 10.3390/molecules23071660. Molecules. 2018. PMID: 29986514 Free PMC article.
-
Similar Active Sites and Mechanisms Do Not Lead to Cross-Promiscuity in Organophosphate Hydrolysis: Implications for Biotherapeutic Engineering.J Am Chem Soc. 2017 Dec 6;139(48):17533-17546. doi: 10.1021/jacs.7b09384. Epub 2017 Nov 21. J Am Chem Soc. 2017. PMID: 29113434 Free PMC article.
References
-
- Kossiakoff AA, Spencer SA. Direct determination of the protonation states of aspartic acid-102 and histidine-57 in the tetrahedral intermediate of the serine proteases: Neutron structure of trypsin. Biochemistry. 1981;20:6462–6474. - PubMed
-
- Wlodawer A, Sjölin L. Structure of ribonuclease A: Results of joint neutron and X-ray refinement at 2.0-A resolution. Biochemistry. 1983;22:2720–2728. - PubMed
-
- Coates L, Erskine PT, Wood SP, Myles DAA, Cooper JB. A neutron Laue diffraction study of endothiapepsin: Implications for the aspartic proteinase mechanism. Biochemistry. 2001;40:13149–13157. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

