The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling
- PMID: 19131965
- PMCID: PMC2657574
- DOI: 10.1038/emboj.2008.285
The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling
Abstract
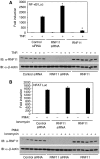
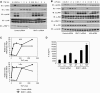
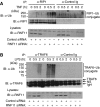
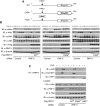
The RING domain protein RNF11 is overexpressed in breast cancers and promotes tumour growth factor-beta (TGF-beta) signalling. RNF11 has been proposed to regulate TGF-beta signalling by interacting with HECT- and SCF-type E3 ligases; however, the role of RNF11 in other signalling pathways is poorly understood. Here, we demonstrate a novel function of RNF11 as a negative regulator of NF-kappaB and jun N-terminal kinase (JNK) signalling pathways. Knockdown of RNF11 with siRNA resulted in persistent tumour necrosis factor (TNF)- and lipopolysaccharide (LPS)-mediated NF-kappaB and JNK signalling. RNF11 interacted with the NF-kappaB inhibitor A20 and its regulatory protein TAX1BP1 in a stimulus-dependent manner. RNF11 negatively regulated RIP1 and TRAF6 ubiquitination upon stimulation with TNF and LPS, respectively. Furthermore, RNF11 was required for A20 to interact with and inactivate RIP1 to inhibit TNF-mediated NF-kappaB activation. Our studies reveal that RNF11, together with TAX1BP1 and Itch, is an essential component of an A20 ubiquitin-editing protein complex that ensures transient activation of inflammatory signalling pathways.
Figures


Comment in
-
RNF11, a new piece in the A20 puzzle.EMBO J. 2009 Mar 4;28(5):455-6. doi: 10.1038/emboj.2009.18. EMBO J. 2009. PMID: 19262463 Free PMC article. No abstract available.
Similar articles
-
Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling.EMBO J. 2007 Sep 5;26(17):3910-22. doi: 10.1038/sj.emboj.7601823. Epub 2007 Aug 16. EMBO J. 2007. PMID: 17703191 Free PMC article.
-
De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling.Nature. 2004 Aug 5;430(7000):694-9. doi: 10.1038/nature02794. Epub 2004 Jul 18. Nature. 2004. PMID: 15258597
-
The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1.Nat Immunol. 2011 Jul 17;12(9):834-43. doi: 10.1038/ni.2066. Nat Immunol. 2011. PMID: 21765415 Free PMC article.
-
Expression, biological activities and mechanisms of action of A20 (TNFAIP3).Biochem Pharmacol. 2010 Dec 15;80(12):2009-20. doi: 10.1016/j.bcp.2010.06.044. Epub 2010 Jul 3. Biochem Pharmacol. 2010. PMID: 20599425 Review.
-
Deubiquitinases in the regulation of NF-κB signaling.Cell Res. 2011 Jan;21(1):22-39. doi: 10.1038/cr.2010.166. Epub 2010 Nov 30. Cell Res. 2011. PMID: 21119682 Free PMC article. Review.
Cited by
-
Genome-wide siRNA screen for mediators of NF-κB activation.Proc Natl Acad Sci U S A. 2012 Feb 14;109(7):2467-72. doi: 10.1073/pnas.1120542109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22308454 Free PMC article.
-
Regulation of NF-κB by deubiquitinases.Immunol Rev. 2012 Mar;246(1):107-24. doi: 10.1111/j.1600-065X.2012.01100.x. Immunol Rev. 2012. PMID: 22435550 Free PMC article. Review.
-
Regulation of NF-κB signaling by the A20 deubiquitinase.Cell Mol Immunol. 2012 Mar;9(2):123-30. doi: 10.1038/cmi.2011.59. Epub 2012 Feb 20. Cell Mol Immunol. 2012. PMID: 22343828 Free PMC article. Review.
-
Tumor necrosis factor (TNF) signaling, but not TWEAK (TNF-like weak inducer of apoptosis)-triggered cIAP1 (cellular inhibitor of apoptosis protein 1) degradation, requires cIAP1 RING dimerization and E2 binding.J Biol Chem. 2010 Jun 4;285(23):17525-36. doi: 10.1074/jbc.M109.087635. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20356846 Free PMC article.
-
Expression, purification and crystallization of the SKICH domain of human TAX1BP1.Acta Crystallogr F Struct Biol Commun. 2014 May;70(Pt 5):619-23. doi: 10.1107/S2053230X14006396. Epub 2014 Apr 15. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24817723 Free PMC article.
References
-
- Anderson LR, Betarbet R, Gearing M, Gulcher J, Hicks AA, Stefansson K, Lah JJ, Levey AI (2007) PARK10 candidate RNF11 is expressed by vulnerable neurons and localizes to Lewy bodies in Parkinson disease brain. J Neuropathol Exp Neurol 66: 955–964 - PubMed
-
- Azmi P, Seth A (2005) RNF11 is a multifunctional modulator of growth factor receptor signalling and transcriptional regulation. Eur J Cancer 41: 2549–2560 - PubMed
-
- Bohgaki M, Tsukiyama T, Nakajima A, Maruyama S, Watanabe M, Koike T, Hatakeyama S (2008) Involvement of Ymer in suppression of NF-κB activation by regulated interaction with lysine-63-linked polyubiquitin chain. Biochim Biophys Acta 1783: 826–837 - PubMed
-
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060 - PubMed
-
- Burger A, Li H, Zhang XK, Pienkowska M, Venanzoni M, Vournakis J, Papas T, Seth A (1998) Breast cancer genome anatomy: correlation of morphological changes in breast carcinomas with expression of the novel gene product Di12. Oncogene 16: 327–333 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

