Differential use of CARD9 by dectin-1 in macrophages and dendritic cells
- PMID: 19124758
- PMCID: PMC2718573
- DOI: 10.4049/jimmunol.182.2.1146
Differential use of CARD9 by dectin-1 in macrophages and dendritic cells
Abstract
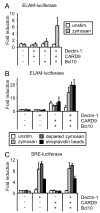
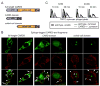
The pattern recognition receptors TLR2 and Dectin-1 play key roles in coordinating the responses of macrophages and dendritic cells (DC) to fungi. Induction of proinflammatory cytokines is instructed by signals from both TLR2 and Dectin-1. A recent report identified a role for CARD9 in innate anti-fungal responses, demonstrating CARD9-Bcl10-mediated activation of NF-kappaB and proinflammatory cytokine induction in murine bone marrow-derived DC stimulated via Dectin-1. We now report that Dectin-1-CARD9 signals fail to activate NF-kappaB and drive TNF-alpha induction in murine bone marrow-derived macrophages. However, priming of bone marrow-derived macrophages with GM-CSF or IFN-gamma permits Dectin-1-CARD9-mediated TNF-alpha induction. Analysis of other macrophage/DC populations revealed further variation in the ability of Dectin-1-CARD9 signaling to drive TNF-alpha production. Resident peritoneal cells and alveolar macrophages produce TNF-alpha upon Dectin-1 ligation, while thioglycollate-elicited peritoneal macrophages and Flt3L-derived DC do not. We present data demonstrating that CARD9 is recruited to phagosomes via its CARD domain where it enhances TLR-induced cytokine production even in cells in which Dectin-1 is insufficient to drive cytokine production. In such cells, Dectin-1, CARD9, and Bcl10 levels are not limiting, and data indicate that these cells express additional factors that restrict Dectin-1-CARD9 signaling for TNF-alpha induction.
Figures




Similar articles
-
C-type lectin receptor dectin-3 mediates trehalose 6,6'-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation.J Biol Chem. 2014 Oct 24;289(43):30052-62. doi: 10.1074/jbc.M114.588574. Epub 2014 Sep 8. J Biol Chem. 2014. PMID: 25202022 Free PMC article.
-
Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells.J Biol Chem. 2009 Mar 13;284(11):7038-46. doi: 10.1074/jbc.M806650200. Epub 2009 Jan 9. J Biol Chem. 2009. PMID: 19136564 Free PMC article.
-
Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity.Nature. 2006 Aug 10;442(7103):651-6. doi: 10.1038/nature04926. Epub 2006 Jul 12. Nature. 2006. PMID: 16862125
-
CARD9 signaling in the innate immune response.Ann N Y Acad Sci. 2008 Nov;1143:35-44. doi: 10.1196/annals.1443.024. Ann N Y Acad Sci. 2008. PMID: 19076343 Review.
-
Dectin-1 and Dectin-2 in innate immunity against fungi.Int Immunol. 2011 Aug;23(8):467-72. doi: 10.1093/intimm/dxr046. Epub 2011 Jun 15. Int Immunol. 2011. PMID: 21677049 Review.
Cited by
-
Impact of Plasma Membrane Domains on IgG Fc Receptor Function.Front Immunol. 2020 Jun 30;11:1320. doi: 10.3389/fimmu.2020.01320. eCollection 2020. Front Immunol. 2020. PMID: 32714325 Free PMC article. Review.
-
Inherited Human BCL10 Deficiencies.J Clin Immunol. 2023 Dec 22;44(1):13. doi: 10.1007/s10875-023-01619-z. J Clin Immunol. 2023. PMID: 38129623 Free PMC article. Review.
-
Nlrp12 deficiency alters gut microbiota and ameliorates Faslpr -mediated systemic autoimmunity in male mice.Front Immunol. 2023 Mar 10;14:1120958. doi: 10.3389/fimmu.2023.1120958. eCollection 2023. Front Immunol. 2023. PMID: 36969209 Free PMC article.
-
Systems Level Dissection of Candida Recognition by Dectins: A Matter of Fungal Morphology and Site of Infection.Pathogens. 2015 Aug 21;4(3):639-61. doi: 10.3390/pathogens4030639. Pathogens. 2015. PMID: 26308062 Free PMC article. Review.
-
Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung.Cell Rep. 2012 Dec 27;2(6):1762-73. doi: 10.1016/j.celrep.2012.10.026. Epub 2012 Nov 29. Cell Rep. 2012. PMID: 23200858 Free PMC article.
References
-
- Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. - PubMed
-
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. - PubMed
-
- Goodridge HS, Underhill DM. Fungal Recognition by TLR2 and Dectin-1. Handb Exp Pharmacol. 2008:87–109. - PubMed
-
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

