Integrins and cell-fate determination
- PMID: 19118209
- PMCID: PMC2714415
- DOI: 10.1242/jcs.018945
Integrins and cell-fate determination
Abstract
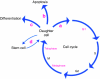
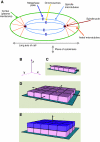
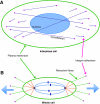
All cellular processes are determined by adhesive interactions between cells and their local microenvironment. Integrins, which constitute one class of cell-adhesion receptor, are multifunctional proteins that link cells to the extracellular matrix and organise integrin adhesion complexes at the cell periphery. Integrin-based adhesions provide anchor points for assembling and organising the cytoskeleton and cell shape, and for orchestrating migration. Integrins also control the fate and function of cells by influencing their proliferation, apoptosis and differentiation. Moreover, new literature demonstrates that integrins control the cell-division axis at mitosis. This extends the influence of integrins over cell-fate decisions, as daughter cells are frequently located in new microenvironments that determine their behaviour following cell division. In this Commentary, I describe how integrins influence cell-fate determination, placing particular emphasis on their role in influencing the direction of cell division and the orientation of the mitotic spindle.
Figures
Similar articles
-
The Role of Mitotic Cell-Substrate Adhesion Re-modeling in Animal Cell Division.Dev Cell. 2018 Apr 9;45(1):132-145.e3. doi: 10.1016/j.devcel.2018.03.009. Dev Cell. 2018. PMID: 29634933
-
Cell shape and intercellular adhesion regulate mitotic spindle orientation.Mol Biol Cell. 2019 Sep 1;30(19):2458-2468. doi: 10.1091/mbc.E19-04-0227. Epub 2019 Aug 14. Mol Biol Cell. 2019. PMID: 31411941 Free PMC article.
-
Spindle orientation and epidermal morphogenesis.Philos Trans R Soc Lond B Biol Sci. 2013 Sep 23;368(1629):20130016. doi: 10.1098/rstb.2013.0016. Print 2013. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24062586 Free PMC article. Review.
-
Mitotic cell responses to substrate topological cues are independent of the molecular nature of adhesion.Sci Signal. 2020 Feb 25;13(620):eaax9940. doi: 10.1126/scisignal.aax9940. Sci Signal. 2020. PMID: 32098802
-
Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor.J Endocrinol. 2011 May;209(2):139-51. doi: 10.1530/JOE-10-0377. Epub 2011 Feb 9. J Endocrinol. 2011. PMID: 21307119 Review.
Cited by
-
Raised mammographic density: causative mechanisms and biological consequences.Breast Cancer Res. 2016 May 3;18(1):45. doi: 10.1186/s13058-016-0701-9. Breast Cancer Res. 2016. PMID: 27142210 Free PMC article. Review.
-
Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation.Development. 2009 Mar;136(6):1019-27. doi: 10.1242/dev.028423. Epub 2009 Feb 11. Development. 2009. PMID: 19211680 Free PMC article.
-
Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation.J Signal Transduct. 2011;2011:738137. doi: 10.1155/2011/738137. Epub 2011 Jul 13. J Signal Transduct. 2011. PMID: 21785723 Free PMC article.
-
Arabidopsis NDR1 is an integrin-like protein with a role in fluid loss and plasma membrane-cell wall adhesion.Plant Physiol. 2011 May;156(1):286-300. doi: 10.1104/pp.110.169656. Epub 2011 Mar 11. Plant Physiol. 2011. PMID: 21398259 Free PMC article.
-
The RhoA-Rok-myosin II pathway is involved in extracellular matrix-mediated regulation of prolactin signaling in mammary epithelial cells.J Cell Physiol. 2012 Apr;227(4):1553-60. doi: 10.1002/jcp.22886. J Cell Physiol. 2012. PMID: 21678418 Free PMC article.
References
-
- Adams, J. C. and Watt, F. M. (1989). Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature 340, 307-309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

