B cells from periodontal disease patients express surface Toll-like receptor 4
- PMID: 19118102
- PMCID: PMC2718806
- DOI: 10.1189/jlb.0708428
B cells from periodontal disease patients express surface Toll-like receptor 4
Abstract
Chronic systemic inflammation links periodontal disease (PD) to increased incidence of cardiovascular disease. Activation of TLRs, particularly TLR4, promotes chronic inflammation in PD by stimulating myeloid cells. B cells from healthy individuals are generally refractory to TLR4 agonists as a result of low surface TLR4 expression. Unexpectedly, a significantly increased percentage of gingival and peripheral blood B cells from patients with PD expressed surface TLR4. Surface expression correlated with an active TLR4 promoter that mimicked the TLR4 promoter in neutrophils. B cells from PD patients were surface myeloid differentiation protein 2-positive and also packaged the enhancer of a proinflammatory cytokine, IL-1 beta, into an active structure, demonstrating that these cells harbor key characteristics of proinflammatory cell types. Furthermore, B cells lacked activating signatures of a natural IL-1 beta inhibitor, IL-1 receptor antagonist. Surprisingly, despite multiple signatures of proinflammatory cells, freshly isolated B cells from PD patients had decreased expression of TLR pathway genes compared with B cells from healthy individuals. Decreases in inflammatory gene expression were even more dramatic in B cells stimulated with a TLR4 ligand from a periodontal pathogen, Porphyromonas gingivalis LPS 1690. In contrast, B cell TLR4 was not activated by the prototypic TLR4 ligand Escherichia coli LPS. These findings raise the unexpected possibility that TLR4 engagement modulates B cell activation in PD patients.
Figures

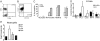
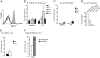
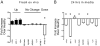

Similar articles
-
Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts.Arch Oral Biol. 2011 Oct;56(10):1064-72. doi: 10.1016/j.archoralbio.2011.02.020. Epub 2011 Mar 31. Arch Oral Biol. 2011. PMID: 21457942
-
Periodontal ligament and gingival fibroblasts from periodontitis patients are more active in interaction with Porphyromonas gingivalis.J Periodontal Res. 2011 Aug;46(4):407-16. doi: 10.1111/j.1600-0765.2011.01353.x. Epub 2011 Feb 17. J Periodontal Res. 2011. PMID: 21332474
-
Global TLR2 and 4 deficiency in mice impacts bone resorption, inflammatory markers and atherosclerosis to polymicrobial infection.Mol Oral Microbiol. 2017 Jun;32(3):211-225. doi: 10.1111/omi.12165. Epub 2016 Jul 20. Mol Oral Microbiol. 2017. PMID: 27224005 Free PMC article.
-
Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review.
-
Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis.Ann Periodontol. 2002 Dec;7(1):72-8. doi: 10.1902/annals.2002.7.1.72. Ann Periodontol. 2002. PMID: 16013219 Review.
Cited by
-
Hemolysis inhibits humoral B-cell responses and modulates alloimmunization risk in patients with sickle cell disease.Blood. 2021 Jan 14;137(2):269-280. doi: 10.1182/blood.2020008511. Blood. 2021. PMID: 33152749 Free PMC article.
-
Toll-like receptors regulate B cell cytokine production in patients with diabetes.Diabetologia. 2010 Jul;53(7):1461-71. doi: 10.1007/s00125-010-1730-z. Epub 2010 Apr 11. Diabetologia. 2010. PMID: 20383694 Free PMC article.
-
Differential regulation of TLR4 expression in human B cells and monocytes.Mol Immunol. 2010 Nov-Dec;48(1-3):82-8. doi: 10.1016/j.molimm.2010.09.008. Epub 2010 Oct 16. Mol Immunol. 2010. PMID: 20956019 Free PMC article.
-
Cytomorphometric Analysis of Inflammation Dynamics in the Periodontium Following the Use of Fixed Dental Prostheses.Molecules. 2020 Oct 12;25(20):4650. doi: 10.3390/molecules25204650. Molecules. 2020. PMID: 33053882 Free PMC article.
-
B cells as under-appreciated mediators of non-auto-immune inflammatory disease.Cytokine. 2010 Jun;50(3):234-42. doi: 10.1016/j.cyto.2010.02.022. Epub 2010 Apr 10. Cytokine. 2010. PMID: 20382544 Free PMC article. Review.
References
-
- Offenbacher S, Beck J D. A perspective on the potential cardioprotective benefits of periodontal therapy. Am Heart J. 2005;149:950–954. - PubMed
-
- Noack B, Genco R J, Trevisan M, Grossi S, Zambon J J, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–1227. - PubMed
-
- Schenkein H A, Barbour S E, Tew J G. Cytokines and inflammatory factors regulating immunoglobulin production in aggressive periodontitis. Periodontol 2000. 2007;45:113–127. - PubMed
-
- Hajishengallis G, Sojar H, Genco R J, DeNardin E. Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immunol Invest. 2004;33:157–172. - PubMed

