Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis
- PMID: 19116365
- PMCID: PMC2630565
- DOI: 10.2353/ajpath.2009.080557
Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis
Abstract
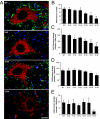
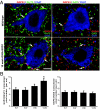
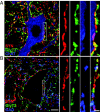
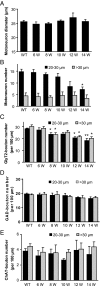
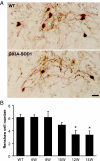
Altered motoneuron excitability is involved in amyotrophic lateral sclerosis pathobiology. To test the hypothesis that inhibitory interneuron innervation of spinal motoneurons is abnormal in an amyotrophic lateral sclerosis mouse model, we measured GABAergic, glycinergic, and cholinergic immunoreactive terminals on spinal motoneurons in mice expressing a mutant form of human superoxide dismutase-1 with a Gly93-->Ala substitution (G93A-SOD1) and in controls at different ages. Glutamic acid decarboxylase, glycine transporter-2, and choline acetyltransferase were used as markers for GABAergic, glycinergic, and cholinergic terminals, respectively. Triple immunofluorescent labeling of boutons contacting motoneurons was visualized by confocal microscopy and analyzed quantitatively. Glycine transporter-2-bouton density on lateral motoneurons was decreased significantly in G93A-SOD1 mice compared with controls. This reduction was absent at 6 weeks of age but present in asymptomatic 8-week-old mice and worsened with disease progression from 12 to 14 weeks of age. Motoneurons lost most glycinergic innervation by 16 weeks of age (end-stage) when there was a significant decrease in the numbers of motoneurons and choline acetyltransferase-positive boutons. No significant differences in glutamic acid decarboxylase-bouton densities were found in G93A-SOD1 mice. Reduction of glycinergic innervation preceded mitochondrial swelling and vacuolization. Calbindin-positive Renshaw cell number was decreased significantly at 12 weeks of age in G93A-SOD1 mice. Thus, either the selective loss of inhibitory glycinergic regulation of motoneuron function or glycinergic interneuron degeneration contributes to motoneuron degeneration in amyotrophic lateral sclerosis.
Figures
Similar articles
-
Altered development in GABA co-release shapes glycinergic synaptic currents in cultured spinal slices of the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.J Physiol. 2016 Jul 1;594(13):3827-40. doi: 10.1113/JP272382. Epub 2016 May 27. J Physiol. 2016. PMID: 27098371 Free PMC article.
-
Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis.J Neurosci. 2011 Feb 23;31(8):2815-27. doi: 10.1523/JNEUROSCI.2475-10.2011. J Neurosci. 2011. PMID: 21414903 Free PMC article.
-
Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis.Mol Neurobiol. 2012 Feb;45(1):30-42. doi: 10.1007/s12035-011-8217-x. Epub 2011 Nov 10. Mol Neurobiol. 2012. PMID: 22072396 Free PMC article. Review.
-
Spinal inhibitory interneuron pathology follows motor neuron degeneration independent of glial mutant superoxide dismutase 1 expression in SOD1-ALS mice.J Neuropathol Exp Neurol. 2011 Aug;70(8):662-77. doi: 10.1097/NEN.0b013e31822581ac. J Neuropathol Exp Neurol. 2011. PMID: 21760539
-
Excitatory action of low frequency depolarizing GABA/glycine synaptic inputs is prevalent in prenatal spinal SOD1G93A motoneurons.J Physiol. 2024 Mar;602(5):913-932. doi: 10.1113/JP285105. Epub 2024 Feb 12. J Physiol. 2024. PMID: 38345477
Cited by
-
Exciting Complexity: The Role of Motor Circuit Elements in ALS Pathophysiology.Front Neurosci. 2020 Jun 17;14:573. doi: 10.3389/fnins.2020.00573. eCollection 2020. Front Neurosci. 2020. PMID: 32625051 Free PMC article. Review.
-
Altered development in GABA co-release shapes glycinergic synaptic currents in cultured spinal slices of the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.J Physiol. 2016 Jul 1;594(13):3827-40. doi: 10.1113/JP272382. Epub 2016 May 27. J Physiol. 2016. PMID: 27098371 Free PMC article.
-
Motoneuron subtypes show specificity in glycine receptor channel abnormalities in a transgenic mouse model of amyotrophic lateral sclerosis.Channels (Austin). 2011 Jul-Aug;5(4):299-303. doi: 10.4161/chan.5.4.16206. Epub 2011 Jul 1. Channels (Austin). 2011. PMID: 21558795 Free PMC article.
-
Reduced Renshaw recurrent inhibition after neonatal sciatic nerve crush in rats.Neural Plast. 2014;2014:786985. doi: 10.1155/2014/786985. Epub 2014 Mar 23. Neural Plast. 2014. PMID: 24778886 Free PMC article.
-
Voltage-gated calcium channels are abnormal in cultured spinal motoneurons in the G93A-SOD1 transgenic mouse model of ALS.Neurobiol Dis. 2016 Sep;93:78-95. doi: 10.1016/j.nbd.2016.04.009. Epub 2016 May 2. Neurobiol Dis. 2016. PMID: 27151771 Free PMC article.
References
-
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. - PubMed
-
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. - PubMed
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahman Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van Den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. - PubMed
-
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

