Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice
- PMID: 19115384
- PMCID: PMC4286201
- DOI: 10.1002/glia.20835
Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice
Abstract
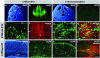
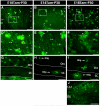
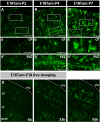
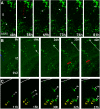
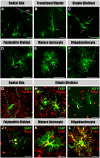
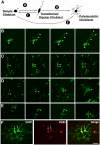
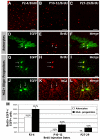
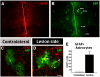
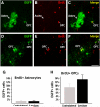
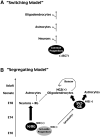
The primary sources of cortical gliogenesis, either during development or after adult brain injury, remain uncertain. We previously generated Nestin-CreER mice to fate-map the progeny of radial glial cells (RG), a source of astrocytes and oligodendrocytes in the nervous system. Here, we show that Nestin-CreER mice label another population of glial progenitors, namely the perinatal subventricular zone (SVZ) glioblasts, if they are crossed with stop-floxed EGFP mice and receive tamoxifen in late embryogenesis (E16-E18). Quantification showed E18 tamoxifen-induction labeled more perinatal SVZ glioblasts than RG and transitional RG combined in the newborn brain (54% vs. 22%). Time-lapse microscopy showed SVZ-glioblasts underwent complex metamorphosis and often-reciprocal transformation into transitional RG. Surprisingly, the E10-dosed RG progenitors produced astrocytes, but no oligodendrocytes, whereas E18-induction fate-mapped both astrocytes and NG2+ oligodendrocyte precursors in the postnatal brain. These results suggest that cortical oligodendrocytes mostly derive from perinatal SVZ glioblast progenitors. Further, by combining genetic fate-mapping and BrdU-labeling, we showed that cortical astrocytes cease proliferation soon after birth (<P10) and only undergo nonproliferative gliosis (i.e., increased GFAP expression without cell-division) after stab-wound injury in adult brains. By contrast, 9.7% of cortical NG2+ progenitors remained mitotic at P29, and the ratio rose to 13.8% after stab-wound injury. Together, these results suggest NG2+ progenitors, rather than GFAP+ astrocytes, are the primary source of proliferative gliosis after adult brain injury.
(c) 2008 Wiley-Liss, Inc.
Figures
Similar articles
-
Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes.J Neurosci Res. 2008 Dec;86(16):3494-502. doi: 10.1002/jnr.21862. J Neurosci Res. 2008. PMID: 18816798
-
Dynamic contribution of nestin-expressing stem cells to adult neurogenesis.J Neurosci. 2007 Nov 14;27(46):12623-9. doi: 10.1523/JNEUROSCI.3812-07.2007. J Neurosci. 2007. PMID: 18003841 Free PMC article.
-
Astrocytes and oligodendrocytes can be generated from NG2+ progenitors after acute brain injury: intracellular localization of oligodendrocyte transcription factor 2 is associated with their fate choice.Eur J Neurosci. 2009 May;29(9):1853-69. doi: 10.1111/j.1460-9568.2009.06736.x. Epub 2009 Apr 28. Eur J Neurosci. 2009. PMID: 19473238
-
Synantocytes: new functions for novel NG2 expressing glia.J Neurocytol. 2002 Jul-Aug;31(6-7):551-65. doi: 10.1023/a:1025751900356. J Neurocytol. 2002. PMID: 14501223 Review.
-
Astrocytes and NG2-glia: what's in a name?J Anat. 2005 Dec;207(6):687-93. doi: 10.1111/j.1469-7580.2005.00489.x. J Anat. 2005. PMID: 16367796 Free PMC article. Review.
Cited by
-
Local generation of glia is a major astrocyte source in postnatal cortex.Nature. 2012 Mar 28;484(7394):376-80. doi: 10.1038/nature10959. Nature. 2012. PMID: 22456708 Free PMC article.
-
Maternal inflammation leads to impaired glutamate homeostasis and up-regulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain.Neurobiol Dis. 2016 Oct;94:116-28. doi: 10.1016/j.nbd.2016.06.010. Epub 2016 Jun 17. Neurobiol Dis. 2016. PMID: 27326668 Free PMC article.
-
Cortical astrocytes develop in a plastic manner at both clonal and cellular levels.Nat Commun. 2019 Oct 25;10(1):4884. doi: 10.1038/s41467-019-12791-5. Nat Commun. 2019. PMID: 31653848 Free PMC article.
-
Acute oligodendrocyte loss with persistent white matter injury in a third trimester equivalent mouse model of fetal alcohol spectrum disorder.Glia. 2017 Aug;65(8):1317-1332. doi: 10.1002/glia.23164. Epub 2017 May 18. Glia. 2017. PMID: 28518477 Free PMC article.
-
The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery.Trends Neurosci. 2012 Apr;35(4):250-60. doi: 10.1016/j.tins.2011.12.005. Epub 2012 Jan 19. Trends Neurosci. 2012. PMID: 22265158 Free PMC article. Review.
References
-
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
-
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
-
- Baracskay KL, Kidd GJ, Miller RH, Trapp BD. NG2-positive cells generate A2B5 positive oligodendrocyte precursor cells. Glia. 2007;55:1001–1010. - PubMed
-
- Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, Rakic P, Kuan CY. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia. Cereb Cortex. 2007;17:2585–2592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

