No exit: targeting the budding process to inhibit filovirus replication
- PMID: 19114059
- PMCID: PMC2666966
- DOI: 10.1016/j.antiviral.2008.12.003
No exit: targeting the budding process to inhibit filovirus replication
Abstract
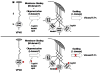
The filoviruses, Ebola and Marburg, cause severe hemorrhagic fever in humans and nonhuman primates, with high mortality rates. Although the filovirus replication pathway is now understood in considerable detail, no antiviral drugs have yet been developed that directly inhibit steps in the replication cycle. One potential target is the filovirus VP40 matrix protein, the key viral protein that drives the budding process, in part by mediating specific virus-host interactions to facilitate the efficient release of virions from the infected cell. This review will summarize current knowledge of key structural and functional domains of VP40 believed to be necessary for efficient budding of virions and virus-like particles. A better understanding of the structure and function of these key regions of VP40 will be crucial, as they may represent novel and rational targets for inhibitors of filovirus egress.
Figures

Similar articles
-
Filovirus proteins for antiviral drug discovery: Structure/function of proteins involved in assembly and budding.Antiviral Res. 2018 Feb;150:183-192. doi: 10.1016/j.antiviral.2017.12.022. Epub 2018 Jan 2. Antiviral Res. 2018. PMID: 29305306 Review.
-
Filovirus proteins for antiviral drug discovery: Structure/function bases of the replication cycle.Antiviral Res. 2017 May;141:48-61. doi: 10.1016/j.antiviral.2017.02.004. Epub 2017 Feb 10. Antiviral Res. 2017. PMID: 28192094 Review.
-
WWOX-Mediated Degradation of AMOTp130 Negatively Affects Egress of Filovirus VP40 Virus-Like Particles.J Virol. 2022 Mar 23;96(6):e0202621. doi: 10.1128/jvi.02026-21. Epub 2022 Feb 2. J Virol. 2022. PMID: 35107375 Free PMC article.
-
Modular mimicry and engagement of the Hippo pathway by Marburg virus VP40: Implications for filovirus biology and budding.PLoS Pathog. 2020 Jan 6;16(1):e1008231. doi: 10.1371/journal.ppat.1008231. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31905227 Free PMC article.
-
Angiomotin Counteracts the Negative Regulatory Effect of Host WWOX on Viral PPxY-Mediated Egress.J Virol. 2021 Mar 25;95(8):e00121-21. doi: 10.1128/JVI.00121-21. Epub 2021 Feb 3. J Virol. 2021. PMID: 33536174 Free PMC article.
Cited by
-
Research progress on the mechanism of exosome-mediated virus infection.Front Cell Infect Microbiol. 2024 Jun 26;14:1418168. doi: 10.3389/fcimb.2024.1418168. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38988816 Free PMC article. Review.
-
Radiolabeled antiviral drugs and antibodies as virus-specific imaging probes.Antiviral Res. 2010 Nov;88(2):129-142. doi: 10.1016/j.antiviral.2010.08.005. Epub 2010 Aug 13. Antiviral Res. 2010. PMID: 20709111 Free PMC article. Review.
-
The life cycle of polyploid giant cancer cells and dormancy in cancer: Opportunities for novel therapeutic interventions.Semin Cancer Biol. 2022 Jun;81:132-144. doi: 10.1016/j.semcancer.2021.10.005. Epub 2021 Oct 17. Semin Cancer Biol. 2022. PMID: 34670140 Free PMC article. Review.
-
Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention.PLoS Pathog. 2015 Oct 29;11(10):e1005220. doi: 10.1371/journal.ppat.1005220. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26513362 Free PMC article.
-
Cysteine Mutations in the Ebolavirus Matrix Protein VP40 Promote Phosphatidylserine Binding by Increasing the Flexibility of a Lipid-Binding Loop.Viruses. 2021 Jul 15;13(7):1375. doi: 10.3390/v13071375. Viruses. 2021. PMID: 34372582 Free PMC article.
References
-
- Ascenzi P, Bocedi A, Heptonstall J, Capobianchi MR, Di Caro A, Mastrangelo E, Bolognesi M, Ippolito G. Ebolavirus and Marburgvirus: insight the Filoviridae family. Mol Aspects Med. 2008;29:151–85. - PubMed
-
- Bausch DG, Sprecher AG, Jeffs B, Boumandouki P. Treatment of Marburg and Ebola hemorrhagic fevers: a strategy for testing new drugs and vaccines under outbreak conditions. Antiviral Res. 2008;78:150–61. - PubMed
-
- Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell. 2008;14:10–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

