Purification of SUMO conjugating enzymes and kinetic analysis of substrate conjugation
- PMID: 19107417
- PMCID: PMC2739043
- DOI: 10.1007/978-1-59745-566-4_11
Purification of SUMO conjugating enzymes and kinetic analysis of substrate conjugation
Abstract
SUMO conjugation to protein substrates requires the concerted action of a dedicated E2 ubiquitin conjugation enzyme (Ubc9) and associated E3 ligases. Although Ubc9 can directly recognize and modify substrate lysine residues that occur within a consensus site for SUMO modification, E3 ligases can redirect specificity and enhance conjugation rates during SUMO conjugation in vitro and in vivo. In this chapter, we will describe methods utilized to purify SUMO conjugating enzymes and model substrates which can be used for analysis of SUMO conjugation in vitro. We will also describe methods to extract kinetic parameters during E3-dependent or E3-independent substrate conjugation.
Figures
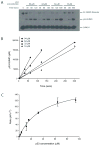
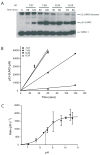
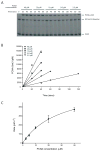
Similar articles
-
Biochemical characterization of SUMO-conjugating enzymes by in vitro sumoylation assays.Methods Enzymol. 2019;618:167-185. doi: 10.1016/bs.mie.2018.12.025. Epub 2019 Feb 11. Methods Enzymol. 2019. PMID: 30850051
-
Role of an N-terminal site of Ubc9 in SUMO-1, -2, and -3 binding and conjugation.Biochemistry. 2003 Aug 26;42(33):9959-69. doi: 10.1021/bi0345283. Biochemistry. 2003. PMID: 12924945
-
Molecular mechanisms in SUMO conjugation.Biochem Soc Trans. 2020 Feb 28;48(1):123-135. doi: 10.1042/BST20190357. Biochem Soc Trans. 2020. PMID: 31872228 Review.
-
Purification and activity assays for Ubc9, the ubiquitin-conjugating enzyme for the small ubiquitin-like modifier SUMO.Methods Enzymol. 2005;398:74-87. doi: 10.1016/S0076-6879(05)98008-7. Methods Enzymol. 2005. PMID: 16275321
-
SUMO: getting it on.Biochem Soc Trans. 2007 Dec;35(Pt 6):1409-13. doi: 10.1042/BST0351409. Biochem Soc Trans. 2007. PMID: 18031233 Review.
Cited by
-
A spectrophotometric assay for conjugation of ubiquitin and ubiquitin-like proteins.Anal Biochem. 2011 Nov 1;418(1):102-10. doi: 10.1016/j.ab.2011.06.034. Epub 2011 Jul 2. Anal Biochem. 2011. PMID: 21771579 Free PMC article.
-
Phage display to identify Nedd8-mimicking peptides as inhibitors of the Nedd8 transfer cascade.Chembiochem. 2013 Jul 22;14(11):1323-30. doi: 10.1002/cbic.201300234. Epub 2013 Jul 3. Chembiochem. 2013. PMID: 23824602 Free PMC article.
-
Strategies to Trap Enzyme-Substrate Complexes that Mimic Michaelis Intermediates During E3-Mediated Ubiquitin-Like Protein Ligation.Methods Mol Biol. 2018;1844:169-196. doi: 10.1007/978-1-4939-8706-1_12. Methods Mol Biol. 2018. PMID: 30242710 Free PMC article.
-
Expression of proteins in Escherichia coli as fusions with maltose-binding protein to rescue non-expressed targets in a high-throughput protein-expression and purification pipeline.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Sep 1;67(Pt 9):1006-9. doi: 10.1107/S1744309111022159. Epub 2011 Aug 13. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 21904041 Free PMC article.
-
A Chemical and Enzymatic Approach to Study Site-Specific Sumoylation.PLoS One. 2015 Dec 3;10(12):e0143810. doi: 10.1371/journal.pone.0143810. eCollection 2015. PLoS One. 2015. PMID: 26633173 Free PMC article.
References
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. - PubMed
-
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. - PubMed
-
- Laney JD, Hochstrasser M. Substrate targeting in the ubiquitin system. Cell. 1999;97:427–430. - PubMed
-
- Melchior F. SUMO--nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

