G2A deficiency in mice promotes macrophage activation and atherosclerosis
- PMID: 19106413
- PMCID: PMC2716803
- DOI: 10.1161/CIRCRESAHA.108.181131
G2A deficiency in mice promotes macrophage activation and atherosclerosis
Abstract
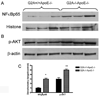
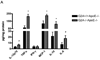
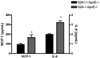
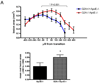
G2A is a stress-inducible G protein-coupled receptor that is expressed on several cell types within atherosclerotic lesions. We demonstrated previously that G2A deficiency in mice increased aortic monocyte recruitment and increased monocyte:endothelial interactions. To investigate the impact of G2A deficiency in macrophages, we isolated peritoneal macrophages from G2A(+/+)ApoE(-/-) and G2A(-/-)ApoE(-/-) mice. G2A(-/-)ApoE(-/-) macrophages had significantly lower apoptosis than control macrophages. The prosurvival genes BCL-2, BCL-xL, and cFLIP were increased in G2A(-/-)ApoE(-/-) macrophages. Macrophages from G2A(-/-)ApoE(-/-) mice also had increased proinflammatory status that was indicative of a M1 macrophage phenotype. This was indicated by significantly increased nuclear translocation of nuclear factor kappaB, as well as production of interleukin-12p40, tumor necrosis factor alpha, and interleukin-6, and reduced expression of arginase-I. Moreover, G2A(-/-)ApoE(-/-) macrophages had reduced ability to engulf apoptotic cells in vitro. We examined atherosclerosis in mice fed a Western diet for 10 weeks and found that G2A deficiency increased lesion size in the aortic root by 50%. Plasma lipid levels were not changed in G2A(-/-)ApoE(-/-) mice. However, we found that absence of G2A increased the number of aortic macrophages and attenuated apoptosis in this cell type. Moreover, bone marrow transplantation studies indicated that deficiency of G2A in marrow-derived cells significantly contributed to atherosclerosis development. In the absence of G2A, increased macrophage activation and decreased apoptosis is associated with accumulation of macrophages in the aorta and increased atherosclerosis.
Figures











Similar articles
-
G2A and LPC: regulatory functions in immunity.Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):73-81. doi: 10.1016/j.prostaglandins.2009.04.007. Epub 2009 Apr 19. Prostaglandins Other Lipid Mediat. 2009. PMID: 19383550 Free PMC article. Review.
-
ApoE-dependent modulation of HDL and atherosclerosis by G2A in LDL receptor-deficient mice independent of bone marrow-derived cells.Arterioscler Thromb Vasc Biol. 2009 Apr;29(4):539-47. doi: 10.1161/ATVBAHA.108.179937. Epub 2009 Jan 22. Arterioscler Thromb Vasc Biol. 2009. PMID: 19164809 Free PMC article.
-
Absence of p21Waf1/Cip1/Sdi1 modulates macrophage differentiation and inflammatory response and protects against atherosclerosis.Circulation. 2004 Dec 21;110(25):3830-41. doi: 10.1161/01.CIR.0000148681.01282.89. Epub 2004 Dec 13. Circulation. 2004. PMID: 15596565
-
Absence of the G protein-coupled receptor G2A in mice promotes monocyte/endothelial interactions in aorta.Circ Res. 2007 Mar 2;100(4):572-80. doi: 10.1161/01.RES.0000258877.57836.d2. Epub 2007 Jan 25. Circ Res. 2007. PMID: 17255525
-
Macrophage death and defective inflammation resolution in atherosclerosis.Nat Rev Immunol. 2010 Jan;10(1):36-46. doi: 10.1038/nri2675. Epub 2009 Dec 4. Nat Rev Immunol. 2010. PMID: 19960040 Free PMC article. Review.
Cited by
-
CD36, but not G2A, modulates efferocytosis, inflammation, and fibrosis following bleomycin-induced lung injury.J Lipid Res. 2013 Apr;54(4):1114-23. doi: 10.1194/jlr.M035352. Epub 2013 Feb 6. J Lipid Res. 2013. PMID: 23393303 Free PMC article.
-
Activation of orphan receptor GPR132 induces cell differentiation in acute myeloid leukemia.Cell Death Dis. 2022 Nov 27;13(11):1004. doi: 10.1038/s41419-022-05434-z. Cell Death Dis. 2022. PMID: 36437247 Free PMC article.
-
G2A and LPC: regulatory functions in immunity.Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):73-81. doi: 10.1016/j.prostaglandins.2009.04.007. Epub 2009 Apr 19. Prostaglandins Other Lipid Mediat. 2009. PMID: 19383550 Free PMC article. Review.
-
The clearance of dying cells: table for two.Cell Death Differ. 2016 Jun;23(6):915-26. doi: 10.1038/cdd.2015.172. Epub 2016 Mar 18. Cell Death Differ. 2016. PMID: 26990661 Free PMC article. Review.
-
Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis.Ther Adv Endocrinol Metab. 2010 Apr;1(2):51-60. doi: 10.1177/2042018810375656. Ther Adv Endocrinol Metab. 2010. PMID: 23148150 Free PMC article.
References
-
- Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. - PubMed
-
- Cybulsky MI, Lichtman AH, Hajra L, Iiyama K. Leukocyte adhesion molecules in atherogenesis. Clin Chim Acta. 1999;286:207–218. - PubMed
-
- Berliner JA, Vora DK, Shih PT. Control of leukocyte adhesion and activation in atherogenesis. In: Pearson J, editor. Vascular Adhesion and Inflammation. Basel, Switzerland: Birkhauser Verlag; 2001.
-
- Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

