Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue
- PMID: 19103590
- PMCID: PMC2645815
- DOI: 10.1074/jbc.M806369200
Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue
Abstract
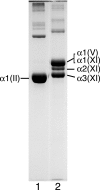
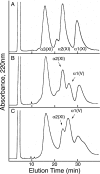
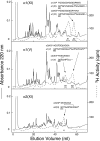
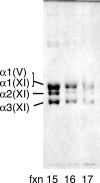
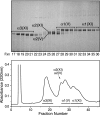
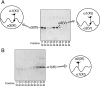
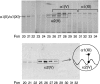
Collagen type V/XI is a minor but essential component of collagen fibrils in vertebrates. We here report on age- and tissue-related variations in isoform usage in cartilages. With maturation of articular cartilage, the alpha1(V) chain progressively replaced the alpha2(XI) chain. A mix of the molecular isoforms, alpha1(XI)alpha1(V)alpha3(XI) and alpha1(XI)alpha2(XI)alpha3(XI), best explained this finding. A prominence of alpha1(V) chains is therefore characteristic and a potential biomarker of mature mammalian articular cartilage. Analysis of cross-linked peptides showed that the alpha1(V) chains were primarily cross-linked to alpha1(XI) chains in the tissue and hence an integral component of the V/XI polymer. From nucleus pulposus of the intervertebral disc (in which the bulk collagen monomer is type II as in articular cartilage), type V/XI collagen consisted of a mix of five genetically distinct chains, alpha1(XI), alpha2(XI), alpha3(XI), alpha1(V), and alpha2(V). These presumably were derived from several different molecular isoforms, including alpha1(XI)alpha2(XI)alpha3(XI), (alpha1(XI))(2)alpha2(V), and others. Meniscal fibrocartilage shows yet another V/XI phenotype. The findings support and extend the concept that the clade B subfamily of COL5 and COL11 gene products should be considered members of the same collagen subfamily, from which, in combination with clade A gene products (COL2A1 or COL5A2), a range of molecular isoforms has evolved into tissue-dependent usage. We propose an evolving role for collagen V/XI isoforms as an adaptable polymeric template of fibril macro-architecture.
Figures

Similar articles
-
Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly.J Biol Chem. 2010 Jan 22;285(4):2580-90. doi: 10.1074/jbc.M109.068726. Epub 2009 Nov 23. J Biol Chem. 2010. PMID: 19940144 Free PMC article.
-
Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse.Matrix Biol. 2007 Oct;26(8):597-603. doi: 10.1016/j.matbio.2007.06.007. Epub 2007 Jul 6. Matrix Biol. 2007. PMID: 17683922 Free PMC article.
-
Molecular properties and fibril ultrastructure of types II and XI collagens in cartilage of mice expressing exclusively the α1(IIA) collagen isoform.Matrix Biol. 2014 Feb;34:105-13. doi: 10.1016/j.matbio.2013.09.006. Epub 2013 Oct 7. Matrix Biol. 2014. PMID: 24113490 Free PMC article.
-
Type V Collagen in Health, Disease, and Fibrosis.Anat Rec (Hoboken). 2016 May;299(5):613-29. doi: 10.1002/ar.23330. Epub 2016 Mar 15. Anat Rec (Hoboken). 2016. PMID: 26910848 Review.
-
Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly.Micron. 2001 Apr;32(3):223-37. doi: 10.1016/s0968-4328(00)00043-3. Micron. 2001. PMID: 11006503 Review.
Cited by
-
Abnormal Bone Collagen Cross-Linking in Osteogenesis Imperfecta/Bruck Syndrome Caused by Compound Heterozygous PLOD2 Mutations.JBMR Plus. 2021 Jan 3;5(3):e10454. doi: 10.1002/jbm4.10454. eCollection 2021 Mar. JBMR Plus. 2021. PMID: 33778323 Free PMC article.
-
Effects of bone matrix proteins on fracture and fragility in osteoporosis.Curr Osteoporos Rep. 2012 Jun;10(2):141-50. doi: 10.1007/s11914-012-0103-6. Curr Osteoporos Rep. 2012. PMID: 22535528 Free PMC article. Review.
-
The cartilage matrisome in adolescent idiopathic scoliosis.Bone Res. 2020 Mar 9;8:13. doi: 10.1038/s41413-020-0089-0. eCollection 2020. Bone Res. 2020. PMID: 32195011 Free PMC article. Review.
-
Biochemical and immuno-histochemical localization of type IIA procollagen in annulus fibrosus of mature bovine intervertebral disc.Matrix Biol Plus. 2021 Jul 5;12:100077. doi: 10.1016/j.mbplus.2021.100077. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34337380 Free PMC article.
-
Characterization of the chondrocyte secretome in photoclickable poly(ethylene glycol) hydrogels.Biotechnol Bioeng. 2017 Sep;114(9):2096-2108. doi: 10.1002/bit.26320. Epub 2017 May 12. Biotechnol Bioeng. 2017. PMID: 28436002 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

