Retrovirally transduced murine T lymphocytes expressing FasL mediate effective killing of prostate cancer cells
- PMID: 19096446
- PMCID: PMC2857530
- DOI: 10.1038/cgt.2008.96
Retrovirally transduced murine T lymphocytes expressing FasL mediate effective killing of prostate cancer cells
Abstract
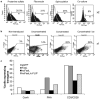
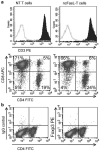
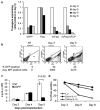
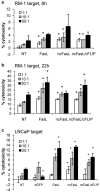
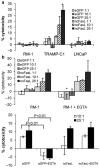
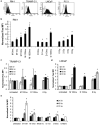
Adoptively transferred T cells possess anticancer activities partially mediated by T-cell FasL engagement of Fas tumor targets. However, antigen-induced T-cell activation and clonal expansion, which stimulates FasL activity, is often inefficient in tumors. As a gene therapy approach to overcome this obstacle, we have created oncoretroviral vectors to overexpress FasL or non-cleavable FasL (ncFasL) on murine T cells of a diverse T-cell receptor repertoire. Expression of c-FLIP was also engineered to prevent apoptosis of transduced cells. Retroviral transduction of murine T lymphocytes has historically been problematic, and we describe optimized T-cell transduction protocols involving CD3/CD28 co-stimulation of T cells, transduction on ice using concentrated oncoretrovirus, and culture with IL-15. Genetically modified T cells home to established prostate cancer tumors in vivo. Co-stimulated T cells expressing FasL, ncFasL and ncFasL/c-FLIP each mediated cytotoxicity in vitro against RM-1 and LNCaP prostate cancer cells. To evaluate the compatibility of this approach with current prostate cancer therapies, we exposed RM-1, LNCaP, and TRAMP-C1 cells to radiation, mitoxantrone, or docetaxel. Fas and H-2(b) expression were upregulated by these methods. We have developed a novel FasL-based immuno-gene therapy for prostate cancer that warrants further investigation given the apparent constitutive and inducible Fas pathway expression in this malignancy.
Figures
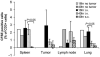
Similar articles
-
Fas-mediated killing of primary prostate cancer cells is increased by mitoxantrone and docetaxel.Mol Cancer Ther. 2008 Sep;7(9):3018-28. doi: 10.1158/1535-7163.MCT-08-0335. Mol Cancer Ther. 2008. PMID: 18790782
-
A novel bystander effect involving tumor cell-derived Fas and FasL interactions following Ad.HSV-tk and Ad.mIL-12 gene therapies in experimental prostate cancer.Gene Ther. 2002 Apr;9(8):511-7. doi: 10.1038/sj.gt.3301669. Gene Ther. 2002. PMID: 11948376
-
Fas ligand is constitutively secreted by prostate cancer cells in vitro.Clin Cancer Res. 1998 Jul;4(7):1803-11. Clin Cancer Res. 1998. PMID: 9676859
-
Dendritic cells transduced with viral interleukin 10 or Fas ligand: no evidence for induction of allotolerance in vivo.Transplantation. 2002 Jan 15;73(1 Suppl):S27-30. doi: 10.1097/00007890-200201151-00010. Transplantation. 2002. PMID: 11810058 Review.
-
On the role and significance of Fas (Apo-1/CD95) ligand (FasL) expression in immune privileged tissues and cancer cells using multiple myeloma as a model.Leuk Lymphoma. 1998 Nov;31(5-6):477-90. doi: 10.3109/10428199809057607. Leuk Lymphoma. 1998. PMID: 9922038 Review.
Cited by
-
Alginate encapsulated cells secreting Fas-ligand reduce lymphoma carcinogenicity.Cancer Sci. 2012 Jan;103(1):116-24. doi: 10.1111/j.1349-7006.2011.02124.x. Epub 2011 Nov 27. Cancer Sci. 2012. PMID: 22017300 Free PMC article.
-
Killer artificial antigen-presenting cells: the synthetic embodiment of a 'guided missile'.Immunotherapy. 2010 Jul;2(4):539-50. doi: 10.2217/imt.10.26. Immunotherapy. 2010. PMID: 20636007 Free PMC article. Review.
References
-
- Gade TPF, Hassen W, Santos E, Gunset G, Saudemont A, Gong MC, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005;65:9080–9088. - PubMed
-
- Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–940. - PubMed
-
- Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15:70–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

