Use of copper and insulin-resistance to accelerate cognitive deficits and synaptic protein loss in a rat Abeta-infusion Alzheimer's disease model
- PMID: 19096161
- PMCID: PMC4313743
- DOI: 10.3233/jad-2008-15409
Use of copper and insulin-resistance to accelerate cognitive deficits and synaptic protein loss in a rat Abeta-infusion Alzheimer's disease model
Abstract
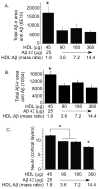
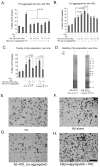
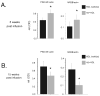
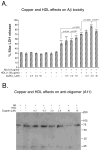
The rat amyloid-beta (Abeta) intracerebroventricular infusion can model aspects of Alzheimer's disease (AD) and has predicted efficacy of therapies such as ibuprofen and curcumin in transgenic mouse models. High density lipoprotein (HDL), a normal plasma carrier of Abeta, is used to attenuate Abeta aggregation within the pump, causing Abeta-dependent toxicity and cognitive deficits within 3 months. Our goal was to identify factors that might accelerate onset of Abeta-dependent deficits to improve efficiency and cost-effectiveness of model. We focused on: 1) optimizing HDL-Abeta preparation for maximal toxicity; 2) evaluating the role of copper, a factor typically in water that can impact oligomer stability; and 3) determining impact of insulin resistance (type II diabetes), a risk factor for AD. In vitro studies were performed to determine doses of copper and methods of Abeta-HDL preparation that maximized toxicity. These preparations when infused resulted in earlier onset of cognitive deficits within 6 weeks post-infusion. Induction of insulin resistance did not exacerbate Abeta-dependent cognitive deficits, but did exacerbate synaptic protein loss. In summary, the newly described in vivo infusion model may be useful cost-effective method for screening for new therapeutic drugs for AD.
Conflict of interest statement
The authors state that there are no financial conflicts of interests.
Figures

Similar articles
-
RAPGEF2 mediates oligomeric Aβ-induced synaptic loss and cognitive dysfunction in the 3xTg-AD mouse model of Alzheimer's disease.Neuropathol Appl Neurobiol. 2021 Aug;47(5):625-639. doi: 10.1111/nan.12686. Epub 2021 Jan 6. Neuropathol Appl Neurobiol. 2021. PMID: 33345400 Free PMC article.
-
Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits.PLoS One. 2014 Nov 12;9(11):e111898. doi: 10.1371/journal.pone.0111898. eCollection 2014. PLoS One. 2014. PMID: 25390368 Free PMC article.
-
Aβ mediates F-actin disassembly in dendritic spines leading to cognitive deficits in Alzheimer's disease.J Neurosci. 2018 Jan 31;38(5):1085-1099. doi: 10.1523/JNEUROSCI.2127-17.2017. Epub 2017 Dec 15. J Neurosci. 2018. PMID: 29246925 Free PMC article.
-
Systematic review of the relationship between amyloid-β levels and measures of transgenic mouse cognitive deficit in Alzheimer's disease.J Alzheimers Dis. 2015;44(3):787-95. doi: 10.3233/JAD-142208. J Alzheimers Dis. 2015. PMID: 25362040 Free PMC article. Review.
-
Drebrin in Alzheimer's Disease.Adv Exp Med Biol. 2017;1006:203-223. doi: 10.1007/978-4-431-56550-5_12. Adv Exp Med Biol. 2017. PMID: 28865022 Review.
Cited by
-
Interactions between Aβ oligomers and presynaptic cholinergic signaling: age-dependent effects on attentional capacities.Behav Brain Res. 2014 Nov 1;274:30-42. doi: 10.1016/j.bbr.2014.07.046. Epub 2014 Aug 4. Behav Brain Res. 2014. PMID: 25101540 Free PMC article.
-
Changes in insulin-signaling transduction pathway underlie learning/memory deficits in an Alzheimer's disease rat model.J Neural Transm (Vienna). 2012 Nov;119(11):1407-16. doi: 10.1007/s00702-012-0803-1. Epub 2012 Apr 17. J Neural Transm (Vienna). 2012. PMID: 22527777
-
Role of insulin resistance in Alzheimer's disease.Metab Brain Dis. 2015 Aug;30(4):839-51. doi: 10.1007/s11011-014-9631-3. Epub 2014 Nov 16. Metab Brain Dis. 2015. PMID: 25399337 Review.
-
GSK3 inhibitors show benefits in an Alzheimer's disease (AD) model of neurodegeneration but adverse effects in control animals.Neurobiol Dis. 2009 Feb;33(2):193-206. doi: 10.1016/j.nbd.2008.10.007. Epub 2008 Nov 5. Neurobiol Dis. 2009. PMID: 19038340 Free PMC article.
References
-
- Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling KQ, Huang X, Moir RD, Wang D, Sayre LM, Smith MA, Chen SG, Bush AI. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry. 2004;43:560–568. - PubMed
-
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puolivali J, Lesne S, Ashe KH, Muchowski PJ, Mucke L. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. - PubMed
-
- Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. - PubMed
-
- Cleary J, Hittner JM, Semotuk M, Mantyh P, O’Hare E. b-amyloid (1-40) effects on behavior and memory. Brain Res. 1995;682:69–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

