NOD-like receptors and inflammation
- PMID: 19090963
- PMCID: PMC2656221
- DOI: 10.1186/ar2525
NOD-like receptors and inflammation
Abstract
The nucleotide-binding and oligomerization domain, leucine-rich repeat (also known as NOD-like receptors, both abbreviated to NLR) family of intracellular pathogen recognition receptors are increasingly being recognized to play a pivotal role in the pathogenesis of a number of rare monogenic diseases, as well as some more common polygenic conditions. Bacterial wall constituents and other cellular stressor molecules are recognized by a range of NLRs, which leads to activation of the innate immune response and upregulation of key proinflammatory pathways, such as IL-1beta production and translocation of nuclear factor-kappaB to the nucleus. These signalling pathways are increasingly being targeted as potential sites for new therapies. This review discusses the role played by NLRs in a variety of inflammatory diseases and describes the remarkable success to date of these therapeutic agents in treating some of the disorders associated with aberrant NLR function.
Figures
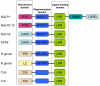


Similar articles
-
Unleashing the therapeutic potential of NOD-like receptors.Nat Rev Drug Discov. 2009 Jun;8(6):465-79. doi: 10.1038/nrd2783. Nat Rev Drug Discov. 2009. PMID: 19483708 Review.
-
Function of Nod-like receptors in microbial recognition and host defense.Immunol Rev. 2009 Jan;227(1):106-28. doi: 10.1111/j.1600-065X.2008.00734.x. Immunol Rev. 2009. PMID: 19120480 Free PMC article. Review.
-
NOD-like receptors: role in innate immunity and inflammatory disease.Annu Rev Pathol. 2009;4:365-98. doi: 10.1146/annurev.pathol.4.110807.092239. Annu Rev Pathol. 2009. PMID: 18928408 Review.
-
Signal transduction pathways used by NLR-type innate immune receptors.Mol Biosyst. 2008 May;4(5):380-6. doi: 10.1039/b718948f. Epub 2008 Mar 27. Mol Biosyst. 2008. PMID: 18414735 Review.
-
NLR, the nucleotide-binding domain leucine-rich repeat containing gene family.Curr Opin Immunol. 2008 Feb;20(1):3-9. doi: 10.1016/j.coi.2008.01.003. Epub 2008 Feb 15. Curr Opin Immunol. 2008. PMID: 18280719 Review.
Cited by
-
Missense mutations in the MEFV gene are associated with fibromyalgia syndrome and correlate with elevated IL-1beta plasma levels.PLoS One. 2009 Dec 30;4(12):e8480. doi: 10.1371/journal.pone.0008480. PLoS One. 2009. PMID: 20041150 Free PMC article.
-
Inflammation: canakinumab for the cryopyrin-associated periodic syndromes.Nat Rev Rheumatol. 2009 Oct;5(10):529-30. doi: 10.1038/nrrheum.2009.195. Nat Rev Rheumatol. 2009. PMID: 19798026 No abstract available.
-
Ultrasonographic assessment reveals detailed distribution of synovial inflammation in Blau syndrome.Arthritis Res Ther. 2014 Apr 8;16(2):R89. doi: 10.1186/ar4533. Arthritis Res Ther. 2014. PMID: 24713464 Free PMC article.
-
NLRP10 promotes AGEs-induced NLRP1 and NLRP3 inflammasome activation via ROS/MAPK/NF-κB signaling in human periodontal ligament cells.Odontology. 2024 Jan;112(1):100-111. doi: 10.1007/s10266-023-00813-0. Epub 2023 Apr 12. Odontology. 2024. PMID: 37043073
-
Hyperoxia Provokes Time- and Dose-Dependent Gut Injury and Endotoxemia and Alters Gut Microbiome and Transcriptome in Mice.Front Med (Lausanne). 2021 Nov 17;8:732039. doi: 10.3389/fmed.2021.732039. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34869425 Free PMC article.
References
-
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an inter-leukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. - DOI - PubMed
-
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. - PubMed
-
- Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

