SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis
- PMID: 19088199
- PMCID: PMC2634889
- DOI: 10.1073/pnas.0810278105
SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis
Abstract
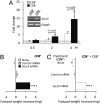
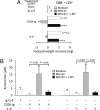
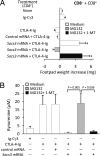
Despite their common ability to activate intracellular signaling through CD80/CD86 molecules, cytotoxic T lymphocyte antigen 4 (CTLA-4)-Ig and CD28-Ig bias the downstream response in opposite directions, the latter promoting immunity, and CTLA-4-Ig tolerance, in dendritic cells (DCs) with opposite but flexible programs of antigen presentation. Nevertheless, in the absence of suppressor of cytokine signaling 3 (SOCS3), CD28-Ig-and the associated, dominant IL-6 response-become immunosuppressive and mimic the effect of CTLA-4-Ig, including a high functional expression of the tolerogenic enzyme indoleamine 2,3-dioxygenase (IDO). Here we show that forced SOCS3 expression antagonized CTLA-4-Ig activity in a proteasome-dependent fashion. Unrecognized by previous studies, IDO appeared to possess two tyrosine residues within two distinct putative immunoreceptor tyrosine-based inhibitory motifs, VPY(115)CEL and LLY(253)EGV. We found that SOCS3-known to interact with phosphotyrosine-containing peptides and be selectively induced by CD28-Ig/IL-6-would bind IDO and target the IDO/SOCS3 complex for ubiquitination and subsequent proteasomal degradation. This event accounted for the ability of CD28-Ig and IL-6 to convert otherwise tolerogenic, IDO-competent DCs into immunogenic cells. Thus onset of immunity in response to antigen within an early inflammatory context requires that IDO be degraded in tolerogenic DCs. In addition to identifying SOCS3 as a candidate signature for mouse DC subsets programmed to direct immunity, this study demonstrates that IDO undergoes regulatory proteolysis in response to immunogenic stimuli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Proteasomal Degradation of Indoleamine 2,3-Dioxygenase in CD8 Dendritic Cells is Mediated by Suppressor of Cytokine Signaling 3 (SOCS3).Int J Tryptophan Res. 2010;3:91-7. doi: 10.4137/ijtr.s3971. Epub 2010 Jun 10. Int J Tryptophan Res. 2010. PMID: 22084591 Free PMC article.
-
Cutting edge: silencing suppressor of cytokine signaling 3 expression in dendritic cells turns CD28-Ig from immune adjuvant to suppressant.J Immunol. 2005 Jun 1;174(11):6582-6. doi: 10.4049/jimmunol.174.11.6582. J Immunol. 2005. PMID: 15905495
-
Ligand and cytokine dependence of the immunosuppressive pathway of tryptophan catabolism in plasmacytoid dendritic cells.Int Immunol. 2005 Nov;17(11):1429-38. doi: 10.1093/intimm/dxh321. Epub 2005 Sep 19. Int Immunol. 2005. PMID: 16172135
-
T cell regulatory plasmacytoid dendritic cells expressing indoleamine 2,3 dioxygenase.Handb Exp Pharmacol. 2009;(188):165-96. doi: 10.1007/978-3-540-71029-5_8. Handb Exp Pharmacol. 2009. PMID: 19031026 Free PMC article. Review.
-
Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity.Immunol Invest. 2012;41(6-7):738-64. doi: 10.3109/08820139.2012.676122. Immunol Invest. 2012. PMID: 23017144 Free PMC article. Review.
Cited by
-
Docking Studies and Molecular Dynamic Simulations Reveal Different Features of IDO1 Structure.Mol Inform. 2016 Sep;35(8-9):449-59. doi: 10.1002/minf.201501038. Epub 2016 Jul 19. Mol Inform. 2016. PMID: 27546049 Free PMC article.
-
Regulation of indoleamine 2,3-dioxygenase in primary human saphenous vein endothelial cells.J Inflamm Res. 2015 May 21;8:97-106. doi: 10.2147/JIR.S82202. eCollection 2015. J Inflamm Res. 2015. PMID: 26056484 Free PMC article.
-
Indoleamine 2,3-dioxygenase 1 activation in mature cDC1 promotes tolerogenic education of inflammatory cDC2 via metabolic communication.Immunity. 2022 Jun 14;55(6):1032-1050.e14. doi: 10.1016/j.immuni.2022.05.013. Immunity. 2022. PMID: 35704993 Free PMC article.
-
The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells.J Immunol. 2014 Feb 1;192(3):1231-40. doi: 10.4049/jimmunol.1300720. Epub 2014 Jan 3. J Immunol. 2014. PMID: 24391212 Free PMC article.
-
Inhibition of the BTK-IDO-mTOR axis promotes differentiation of monocyte-lineage dendritic cells and enhances anti-tumor T cell immunity.Immunity. 2021 Oct 12;54(10):2354-2371.e8. doi: 10.1016/j.immuni.2021.09.005. Epub 2021 Oct 5. Immunity. 2021. PMID: 34614413 Free PMC article.
References
-
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. - PubMed
-
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. - PubMed
-
- Grohmann U, et al. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol. 2003;171:2581–2587. - PubMed
-
- Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. - PubMed
-
- Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

