Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury
- PMID: 19088177
- PMCID: PMC2653946
- DOI: 10.1189/jlb.0808491
Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury
Abstract
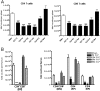
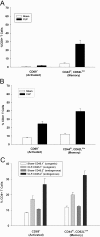
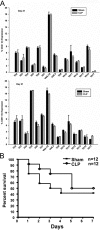
Sepsis is a severe, life-threatening infection and a leading cause of death in hospitals. A hallmark of sepsis is the profound apoptosis-induced depletion of lymphocytes generating a lymphopenic environment. As lymphopenia can induce nonantigen-driven homeostatic proliferation (HP), we examined this process during sepsis. CD4(+) and CD8(+) T cells, which were depleted within 24 h of sepsis induction, remained at significantly reduced levels until Day 21 when normal numbers were detected. When HP was examined, naïve CD8(+) T cells proliferated between Day 7 and Day 21 post-cecal ligation and puncture, developing into memory cells with relatively few cells expressing an activation phenotype. Conversely, naïve CD4(+) T cells did not undergo HP, but proportionally higher numbers expressed activation markers. Adoptive transfer studies revealed that T cells from mice that had recovered from sepsis were not protective when transferred to naïve mice undergoing sepsis. In addition, the TCR repertoire was not skewed toward any specific Vbeta type but resembled the repertoire found in normal mice, suggesting that T cells were not primed to antigens resulting from the infection. Interestingly, depletion of endogenous CD8(+) but not CD4(+) T cells restored the ability of naive CD4(+) T cells to undergo HP, increasing the number of CD4(+) T cells with memory but not activation markers. We conclude that homeostatic control in the postseptic environment permits recovery of the T cell repertoire to normal levels without generating antigen-specific memory or aberrant T cell specificities. Restoration of homeostatic control mechanisms might be a rational therapy for this disorder.
Figures

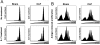


Similar articles
-
NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells.J Immunol. 2010 Jun 15;184(12):6649-57. doi: 10.4049/jimmunol.0903729. Epub 2010 May 14. J Immunol. 2010. PMID: 20483732
-
Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions.Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19414-9. doi: 10.1073/pnas.0807743105. Epub 2008 Nov 25. Proc Natl Acad Sci U S A. 2008. PMID: 19033460 Free PMC article.
-
The nature of the lymphopenic environment dictates protective function of homeostatic-memory CD8+ T cells.Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18484-9. doi: 10.1073/pnas.0806487105. Epub 2008 Nov 19. Proc Natl Acad Sci U S A. 2008. PMID: 19020089 Free PMC article.
-
Cytokine signals in T-cell homeostasis.J Immunother. 2005 Jul-Aug;28(4):289-94. doi: 10.1097/01.cji.0000165356.03924.e7. J Immunother. 2005. PMID: 16000945 Review.
-
Endogenous proliferation: burst-like CD4 T cell proliferation in lymphopenic settings.Semin Immunol. 2005 Jun;17(3):201-7. doi: 10.1016/j.smim.2005.02.005. Semin Immunol. 2005. PMID: 15826825 Review.
Cited by
-
Research progress of biomarkers in evaluating the severity and prognostic value of severe pneumonia in children.Front Pediatr. 2024 Oct 1;12:1417644. doi: 10.3389/fped.2024.1417644. eCollection 2024. Front Pediatr. 2024. PMID: 39411281 Free PMC article. Review.
-
Lymphopenia in sepsis: a narrative review.Crit Care. 2024 Sep 20;28(1):315. doi: 10.1186/s13054-024-05099-4. Crit Care. 2024. PMID: 39304908 Free PMC article. Review.
-
Defining Parameters That Modulate Susceptibility and Protection to Respiratory Murine Coronavirus MHV1 Infection.J Immunol. 2024 Feb 15;212(4):563-575. doi: 10.4049/jimmunol.2300434. J Immunol. 2024. PMID: 38149923
-
Regulation of inflammation and immunity in sepsis by E3 ligases.Front Endocrinol (Lausanne). 2023 Jul 3;14:1124334. doi: 10.3389/fendo.2023.1124334. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37465127 Free PMC article. Review.
-
Sublethal whole-body irradiation induces permanent loss and dysfunction in pathogen-specific circulating memory CD8 T cell populations.Proc Natl Acad Sci U S A. 2023 Jul 4;120(27):e2302785120. doi: 10.1073/pnas.2302785120. Epub 2023 Jun 26. Proc Natl Acad Sci U S A. 2023. PMID: 37364124 Free PMC article.
References
-
- Angus D C, Linde-Zwirble W T, Lidicker J, Clermont G, Carcillo J, Pinsky M R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Murphy S L. Deaths: final data for 1998. Natl Vital Stat Rep. 2000;48:1–105. - PubMed
-
- Hotchkiss R S, Swanson P E, Freeman B D, Tinsley K W, Cobb J P, Matuschak G M, Buchman T G, Karl I E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. - PubMed
-
- Hotchkiss R S, Karl I E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. - PubMed
-
- Hotchkiss R S, Tinsley K W, Swanson P E, Grayson M H, Osborne D F, Wagner T H, Cobb J P, Coopersmith C, Karl I E. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

