Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells
- PMID: 19088029
- PMCID: PMC4403242
- DOI: 10.1158/1078-0432.CCR-08-1539
Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells
Abstract
Purpose: Nuclear factor kappaB (NFkappaB) activity may increase survival and protect cancer cells from chemotherapy. Therefore, NFkappaB activity may be prognostic, and inhibition of NFkappaB may be useful for pancreatic cancer therapy. To test these hypotheses, we examined NFkappaB activity and the effects of inhibiting NFkappaB in several pancreatic cancer cell lines with differing sensitivities to gemcitabine.
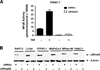
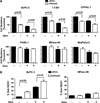
Experimental design: The gemcitabine sensitivity of pancreatic cancer cell lines BxPC-3, L3.6pl, CFPAC-1, MPanc-96, PANC-1, and MIA PaCa-2 were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and fluorescence-activated cell sorting assays. NFkappaB levels were determined by electrophoretic mobility shift assay and reporter assays. The effects of gemcitabine on NFkappaB activity were determined in vitro and in vivo. NFkappaB was inhibited by silencing of the p65/relA subunit using small interfering RNA in vitro and by neutral liposomal delivery of small interfering RNA in vivo, and the effects were evaluated on gemcitabine sensitivity.
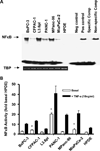
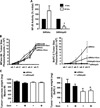
Results: The cell lines L3.6pl, BxPC-3, and CFPAC-1 were sensitive, whereas MPanc-96, PANC-1, and MIA PaCa-2 were resistant to gemcitabine. No significant correlation was observed between basal NFkappaB activity and gemcitabine sensitivity. Gemcitabine treatment did not activate NFkappaB either in vitro or in vivo. Silencing of p65/relA induced apoptosis and increased gemcitabine killing of all gemcitabine-sensitive pancreatic cancer cells. No significant effects, however, were observed on gemcitabine-resistant pancreatic cancer cell lines either in vitro or in vivo.
Conclusions: NFkappaB activity did not correlate with sensitivity to gemcitabine. Silencing of p65/relA was effective alone and in combination with gemcitabine in gemcitabine-sensitive but not gemcitabine-resistant pancreatic cancer cells. Thus, NFkappaB may be a useful therapeutic target for a subset of pancreatic cancers.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
[An experimental study of gemcitabine inducing pancreatic cancer cell apoptosis potentiated by nuclear factor-kappa B P65 siRNA].Zhonghua Wai Ke Za Zhi. 2010 Jan 15;48(2):128-33. Zhonghua Wai Ke Za Zhi. 2010. PMID: 20302733 Chinese.
-
TM4SF1 Promotes Gemcitabine Resistance of Pancreatic Cancer In Vitro and In Vivo.PLoS One. 2015 Dec 28;10(12):e0144969. doi: 10.1371/journal.pone.0144969. eCollection 2015. PLoS One. 2015. PMID: 26709920 Free PMC article.
-
Dimethylamino parthenolide enhances the inhibitory effects of gemcitabine in human pancreatic cancer cells.J Gastrointest Surg. 2012 Jul;16(7):1333-40. doi: 10.1007/s11605-012-1913-7. Epub 2012 May 23. J Gastrointest Surg. 2012. PMID: 22618517
-
Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer.Eur J Pharmacol. 2014 Oct 15;741:8-16. doi: 10.1016/j.ejphar.2014.07.041. Epub 2014 Jul 30. Eur J Pharmacol. 2014. PMID: 25084222 Review.
-
Heat-shock protein 27 plays the key role in gemcitabine-resistance of pancreatic cancer cells.Anticancer Res. 2012 Jun;32(6):2295-9. Anticancer Res. 2012. PMID: 22641665 Review.
Cited by
-
Glycogen synthase kinase 3β: the nexus of chemoresistance, invasive capacity, and cancer stemness in pancreatic cancer.Cancer Drug Resist. 2024 Jan 31;7:4. doi: 10.20517/cdr.2023.84. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38318525 Free PMC article. Review.
-
Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells.Neoplasia. 2010 Sep;12(9):740-7. doi: 10.1593/neo.10576. Neoplasia. 2010. PMID: 20824050 Free PMC article.
-
OXCT1 Enhances Gemcitabine Resistance Through NF-κB Pathway in Pancreatic Ductal Adenocarcinoma.Front Oncol. 2021 Nov 5;11:698302. doi: 10.3389/fonc.2021.698302. eCollection 2021. Front Oncol. 2021. PMID: 34804914 Free PMC article.
-
Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer.Mar Drugs. 2022 May 30;20(6):370. doi: 10.3390/md20060370. Mar Drugs. 2022. PMID: 35736173 Free PMC article. Review.
-
Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions.Cell Rep. 2016 Mar 15;14(10):2325-36. doi: 10.1016/j.celrep.2016.02.029. Epub 2016 Mar 3. Cell Rep. 2016. PMID: 26947075 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- O’Reilly EM, Abou-Alfa GK. Cytotoxic therapy for advanced pancreatic adenocarcinoma. Semin Oncol. 2007;34:347–353. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. - PubMed
-
- Nakanishi C, Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat Rev. 2005;5:297–309. - PubMed
-
- Neumann M, Naumann M. Beyond IκBs: alternative regulation of NF-κB activity. FASEB J. 2007;21:2642–2654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

