Stem cells, phenotypic inversion, and differentiation
- PMID: 19079683
- PMCID: PMC2596332
Stem cells, phenotypic inversion, and differentiation
Abstract
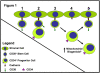
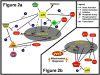
Stem cells possess the potential to cure a myriad of ailments ranging from congenital diseases to illnesses acquired through the physiological process of aging. In the adult, these cells are extremely rare and often difficult to isolate in numbers sufficient to apply to medical treatment. Ex vivo expansion of these cells will be required for most meaningful interventions. The discovery of stem/progenitor cell inversion offers a new avenue for obtaining sufficient numbers of stem cells. Adult progenitor cells are much more common than quiescent stem cells and can be isolated with minimal interventions; therefore, inversion of progenitors to stem cells may become a feasible approach for therapeutic purposes. Stem cells are known to possess few mitochondria, and mitochondrial biogenesis is required for stem cell differentiation. The microtubule cytoskeleton is a major regulator for mitochondrial biogenesis. Investigations in the area of controlling cell differentiation and inducing phenotypic inversion, possibly through manipulation of mitochondrial biogenesis, may contribute to stem cell-based therapies.
Keywords: Stem cells; differentiation; inversion; microtubules; mitochondria.
Figures
Similar articles
-
BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation.Autophagy. 2019 Jul;15(7):1182-1198. doi: 10.1080/15548627.2019.1580095. Epub 2019 Feb 22. Autophagy. 2019. PMID: 30741592 Free PMC article.
-
A Conserved Role for Asrij/OCIAD1 in Progenitor Differentiation and Lineage Specification Through Functional Interaction With the Regulators of Mitochondrial Dynamics.Front Cell Dev Biol. 2021 Jul 6;9:643444. doi: 10.3389/fcell.2021.643444. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34295888 Free PMC article.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
To breathe or not to breathe: the haematopoietic stem/progenitor cells dilemma.Br J Pharmacol. 2013 Aug;169(8):1652-71. doi: 10.1111/bph.12253. Br J Pharmacol. 2013. PMID: 23714011 Free PMC article. Review.
-
Ex vivo expansion of hematopoietic cells and their clinical use.Haematologica. 1998 Sep;83(9):824-48. Haematologica. 1998. PMID: 9825579 Review.
Cited by
-
Mitochondrial DNA and inflammatory diseases.Hum Genet. 2012 Feb;131(2):161-73. doi: 10.1007/s00439-011-1057-y. Epub 2011 Jul 7. Hum Genet. 2012. PMID: 21735170 Review.
-
Differentiation and regeneration potential of mesenchymal progenitor cells derived from traumatized muscle tissue.J Cell Mol Med. 2011 Nov;15(11):2377-88. doi: 10.1111/j.1582-4934.2010.01225.x. J Cell Mol Med. 2011. PMID: 21129154 Free PMC article.
-
Stem cells and neuroprotection: understanding the players.Int J Mol Sci. 2010 Sep 15;11(9):3288-97. doi: 10.3390/ijms11093288. Int J Mol Sci. 2010. PMID: 20957094 Free PMC article. Review.
-
Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells.Eur J Dent. 2014 Jul;8(3):307-313. doi: 10.4103/1305-7456.137631. Eur J Dent. 2014. PMID: 25202208 Free PMC article.
-
Physiologically normal 5% O2 supports neuronal differentiation and resistance to inflammatory injury in neural stem cell cultures.J Neurosci Res. 2015 Nov;93(11):1703-12. doi: 10.1002/jnr.23615. Epub 2015 Jul 3. J Neurosci Res. 2015. PMID: 26147710 Free PMC article.
References
-
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896–902. - PubMed
-
- Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Nakamura T, Eguchi H, Kawano S. The transdifferentiation of bone-marrow-derived cells in colonic mucosal regeneration after dextran-sulfate-sodium-induced colitis in mice. Pharmacology. 2007;80:193–9. - PubMed
-
- Udani VM. The continuum of stem cell transdifferentiation: possibility of hematopoietic stem cell plasticity with concurrent CD45 expression. Stem Cells Dev. 2006;15:1–3. - PubMed
-
- Ikeda T. 2007. Stem cells and neonatal brain injury. Cell Tissue Res (In Press)
-
- Song SJ, Jeon O, Yang HS, Han DK, Kim BS. Effects of culture conditions on osteogenic differentiation in human mesenchymal stem cells. J Microbiol Biotechnol. 2007;17:1113–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
