Mitochondrial and nuclear cross talk in cell death: parthanatos
- PMID: 19076445
- PMCID: PMC4454457
- DOI: 10.1196/annals.1427.014
Mitochondrial and nuclear cross talk in cell death: parthanatos
Abstract
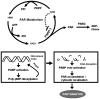
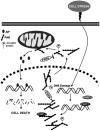
Poly(ADP-ribose) polymerase-1 (PARP-1) is an abundant nuclear protein best known to facilitate DNA base excision repair. Recent work has expanded the physiologic functions of PARP-1, and it is clear that the full range of biologic actions of this important protein are not yet fully understood. Regulation of the product of PARP-1, poly(ADP-ribose) (PAR), is a dynamic process with PAR glycohydrolase playing the major role in the degradation of the polymer. Under pathophysiologic situations overactivation of PARP-1 results in unregulated PAR synthesis and widespread neuronal cell death. Once thought to be necrotic cell death resulting from energy failure, we have found that PARP-1-dependent cell death is dependent on the generation of PAR, which triggers the nuclear translocation of apoptosis-inducing factor resulting in caspase-independent cell death. This form of cell death is distinct from apoptosis, necrosis, or autophagy and is termed parthanatos. PARP-1-dependent cell death has been implicated in tissues throughout the body and in diseases afflicting hundreds of millions worldwide, including stroke, Parkinson's disease, heart attack, diabetes, and ischemia reperfusion injury in numerous tissues. The breadth of indications for PARP-1 injury make parthanatos a clinically important form of cell death to understand and control.
Figures
Similar articles
-
Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities.Br J Pharmacol. 2014 Apr;171(8):2000-16. doi: 10.1111/bph.12416. Br J Pharmacol. 2014. PMID: 24684389 Free PMC article. Review.
-
Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos.Exp Neurol. 2009 Aug;218(2):193-202. doi: 10.1016/j.expneurol.2009.03.020. Epub 2009 Mar 28. Exp Neurol. 2009. PMID: 19332058 Free PMC article. Review.
-
Structure and function of poly(ADP-ribose) polymerase-1: role in oxidative stress-related pathologies.Curr Vasc Pharmacol. 2005 Jul;3(3):209-14. doi: 10.2174/1570161054368625. Curr Vasc Pharmacol. 2005. PMID: 16026317 Review.
-
Mediation of cell death by poly(ADP-ribose) polymerase-1.Pharmacol Res. 2005 Jul;52(1):5-14. doi: 10.1016/j.phrs.2005.02.011. Pharmacol Res. 2005. PMID: 15911329 Review.
-
Parthanatos, a messenger of death.Front Biosci (Landmark Ed). 2009 Jan 1;14(3):1116-28. doi: 10.2741/3297. Front Biosci (Landmark Ed). 2009. PMID: 19273119 Free PMC article. Review.
Cited by
-
Updating experimental models of diabetic cardiomyopathy.J Diabetes Res. 2015;2015:656795. doi: 10.1155/2015/656795. Epub 2015 Apr 20. J Diabetes Res. 2015. PMID: 25973429 Free PMC article. Review.
-
Dopamine quinone modifies and decreases the abundance of the mitochondrial selenoprotein glutathione peroxidase 4.Free Radic Biol Med. 2013 Dec;65:419-427. doi: 10.1016/j.freeradbiomed.2013.06.030. Epub 2013 Jun 28. Free Radic Biol Med. 2013. PMID: 23816523 Free PMC article.
-
Alkyladenine DNA glycosylase deficiency uncouples alkylation-induced strand break generation from PARP-1 activation and glycolysis inhibition.Sci Rep. 2020 Feb 10;10(1):2209. doi: 10.1038/s41598-020-59072-6. Sci Rep. 2020. PMID: 32042007 Free PMC article.
-
The dual role of poly(ADP-ribose) polymerase-1 in modulating parthanatos and autophagy under oxidative stress in rat cochlear marginal cells of the stria vascularis.Redox Biol. 2018 Apr;14:361-370. doi: 10.1016/j.redox.2017.10.002. Epub 2017 Oct 7. Redox Biol. 2018. PMID: 29049980 Free PMC article.
-
Suppression of JNK pathway protects neurons from oxidative injury via attenuating parthanatos in glutamate-treated HT22 neurons.Sci Rep. 2024 Oct 28;14(1):25793. doi: 10.1038/s41598-024-76640-2. Sci Rep. 2024. PMID: 39468165 Free PMC article.
References
-
- Smith S. The world according to PARP. Trends Biochem Sci. 2001;26:174–9. - PubMed
-
- Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004;25:259–64. - PubMed
-
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–93. - PubMed
-
- Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

