Detection of cytomegalovirus in whole blood using three different real-time PCR chemistries
- PMID: 19074593
- PMCID: PMC2607566
- DOI: 10.2353/jmoldx.2009.080073
Detection of cytomegalovirus in whole blood using three different real-time PCR chemistries
Abstract
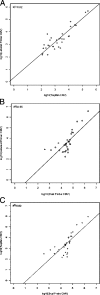
Several different primer-probe chemistries have been produced commercially for real-time PCR detection and quantification of cytomegalovirus, but there are few studies evaluating their relative performance. We assessed three such commercial reagents with respect to analytical and clinical operating characteristics. The samples included 149 clinical whole blood specimens that were de-identified and assayed in parallel with all primer-probe systems. Individual methods used TaqMan, dual fluorescence resonance energy transfer hybridization probes, and labeled primer chemistries. Method comparability was determined both qualitatively, based on pair-wise assessment of concordance, and quantitatively, based on pair-wise linear regression analysis. Analytical sensitivity and the lower end of the linear dynamic range reached 10 target copies per reaction for the TaqMan and labeled primer systems and 100 target copies per reaction for the dual fluorescence resonance energy transfer probe system. Quantitative linearity reached an upper limit of 10(5) copies per reaction for all methods. No assay cross-reactivity was seen with other common viral pathogens (100% analytical specificity). Pair-wise analysis of qualitative results from clinical samples showed no significant differences in sensitivity between the three sets of reagents, and linear regression analysis indicated that the quantitative values achieved were comparable in all positive specimens. The findings demonstrate that similar analytical and clinical performance characteristics can be demonstrated for quantitative detection of cytomegalovirus in clinical whole blood extracts using a wide variety of real-time PCR chemistries.
Figures

Similar articles
-
Comparison of quantitative competitive polymerase chain reaction-enzyme-linked immunosorbent assay with LightCycler-based polymerase chain reaction for measuring cytomegalovirus DNA in patients after hematopoietic stem cell transplantation.Diagn Microbiol Infect Dis. 2006 Feb;54(2):115-20. doi: 10.1016/j.diagmicrobio.2005.08.021. Epub 2006 Jan 9. Diagn Microbiol Infect Dis. 2006. PMID: 16406183
-
Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR.J Clin Microbiol. 2004 Mar;42(3):1142-8. doi: 10.1128/JCM.42.3.1142-1148.2004. J Clin Microbiol. 2004. PMID: 15004066 Free PMC article.
-
[Evaluation of Biosewoom Real-Q Cytomegalovirus Quantification kit for Cytomegalovirus viral load measure].Korean J Lab Med. 2007 Aug;27(4):298-304. doi: 10.3343/kjlm.2007.27.4.298. Korean J Lab Med. 2007. PMID: 18094592 Korean.
-
Quantitation of cytomegalovirus DNA by real-time polymerase chain reaction in peripheral blood specimens of patients with solid organ transplants: comparison with end-point PCR and pp65 antigen test.J Med Virol. 2006 Jul;78(7):915-22. doi: 10.1002/jmv.20641. J Med Virol. 2006. PMID: 16721848
-
Cytomegalovirus DNA quantification using an automated platform for nucleic acid extraction and real-time PCR assay setup.J Clin Microbiol. 2011 Jul;49(7):2703-5. doi: 10.1128/JCM.00721-11. Epub 2011 May 11. J Clin Microbiol. 2011. PMID: 21562101 Free PMC article.
Cited by
-
Establishment and application of a point-of-care testing and diagnosis method for early immediate expression gene IE1 of cytomegalovirus in maternal urine based on isothermal amplification.Virus Res. 2023 Nov;337:199229. doi: 10.1016/j.virusres.2023.199229. Epub 2023 Oct 7. Virus Res. 2023. PMID: 37769815 Free PMC article.
-
Rapid quantitation of cytomegalovirus DNA in whole blood by a new molecular assay based on automated sample preparation and real-time PCR.Med Microbiol Immunol. 2010 Nov;199(4):311-6. doi: 10.1007/s00430-010-0164-z. Epub 2010 Jun 18. Med Microbiol Immunol. 2010. PMID: 20559848
-
Microelectromechanical system-based biosensor for label-free detection of human cytomegalovirus.IET Nanobiotechnol. 2023 Feb;17(1):32-39. doi: 10.1049/nbt2.12109. Epub 2022 Dec 20. IET Nanobiotechnol. 2023. PMID: 36537882 Free PMC article.
-
Comparative evaluation of three automated systems for DNA extraction in conjunction with three commercially available real-time PCR assays for quantitation of plasma Cytomegalovirus DNAemia in allogeneic stem cell transplant recipients.J Clin Microbiol. 2011 Aug;49(8):2899-904. doi: 10.1128/JCM.00785-11. Epub 2011 Jun 22. J Clin Microbiol. 2011. PMID: 21697323 Free PMC article.

