Attenuation of rabies virus replication and virulence by picornavirus internal ribosome entry site elements
- PMID: 19073737
- PMCID: PMC2643788
- DOI: 10.1128/JVI.02055-08
Attenuation of rabies virus replication and virulence by picornavirus internal ribosome entry site elements
Abstract
Gene expression of nonsegmented negative-strand RNA viruses is regulated at the transcriptional level and relies on the canonical 5'-end-dependent translation of capped viral mRNAs. Here, we have used internal ribosome entry sites (IRES) from picornaviruses to control the expression level of the phosphoprotein P of the neurotropic rabies virus (RV; Rhabdoviridae), which is critically required for both viral replication and escape from the host interferon response. In a dual luciferase reporter RV, the IRES elements of poliovirus (PV) and human rhinovirus type 2 (HRV2) were active in a variety of cell lines from different host species. While a generally lower activity of the HRV2 IRES was apparent compared to the PV IRES, specific deficits of the HRV2 IRES in neuronal cell lines were not observed. Recombinant RVs expressing P exclusively from a bicistronic nucleoprotein (N)-IRES-P mRNA showed IRES-specific reduction of replication in cell culture and in neurons of organotypic brain slice cultures, an increased activation of the beta interferon (IFN-beta) promoter, and increased sensitivity to IFN. Intracerebral infection revealed a complete loss of virulence of both PV- and HRV2 IRES-controlled RV for wild-type mice and for transgenic mice lacking a functional IFN-alpha receptor (IFNAR(-/-)). The virulence of HRV2 IRES-controlled RV was most severely attenuated and could be demonstrated only in newborn IFNAR(-/-) mice. Translational control of individual genes is a promising strategy to attenuate replication and virulence of live nonsegmented negative-strand RNA viruses and vectors and to study the function of IRES elements in detail.
Figures
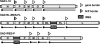


Similar articles
-
Age-dependent poliovirus replication in the mouse central nervous system is determined by internal ribosome entry site-mediated translation.J Virol. 2006 Mar;80(6):2589-95. doi: 10.1128/JVI.80.6.2589-2595.2006. J Virol. 2006. PMID: 16501069 Free PMC article.
-
Genetic dissection of interferon-antagonistic functions of rabies virus phosphoprotein: inhibition of interferon regulatory factor 3 activation is important for pathogenicity.J Virol. 2011 Jan;85(2):842-52. doi: 10.1128/JVI.01427-10. Epub 2010 Nov 17. J Virol. 2011. PMID: 21084487 Free PMC article.
-
The importance of being short: the role of rabies virus phosphoprotein isoforms assessed by differential IRES translation initiation.Eur J Cell Biol. 2012 Jan;91(1):17-23. doi: 10.1016/j.ejcb.2011.01.009. Epub 2011 Mar 12. Eur J Cell Biol. 2012. PMID: 21397980
-
Advances and Breakthroughs in IRES-Directed Translation and Replication of Picornaviruses.mBio. 2023 Apr 25;14(2):e0035823. doi: 10.1128/mbio.00358-23. Epub 2023 Mar 20. mBio. 2023. PMID: 36939331 Free PMC article. Review.
-
Cap-independent translation of picornavirus RNAs: structure and function of the internal ribosomal entry site.Enzyme. 1990;44(1-4):292-309. doi: 10.1159/000468766. Enzyme. 1990. PMID: 1966843 Review.
Cited by
-
Revealing the secrets of neuronal circuits with recombinant rabies virus technology.Front Neural Circuits. 2013 Jan 24;7:2. doi: 10.3389/fncir.2013.00002. eCollection 2013. Front Neural Circuits. 2013. PMID: 23355811 Free PMC article. Review.
-
Reverse genetics of Mononegavirales: How they work, new vaccines, and new cancer therapeutics.Virology. 2015 May;479-480:331-44. doi: 10.1016/j.virol.2015.01.029. Epub 2015 Feb 18. Virology. 2015. PMID: 25702088 Free PMC article. Review.
-
Immunogenicity studies in carnivores using a rabies virus construct with a site-directed deletion in the phosphoprotein.Adv Prev Med. 2011;2011:898171. doi: 10.4061/2011/898171. Epub 2011 Sep 21. Adv Prev Med. 2011. PMID: 21991446 Free PMC article.
-
Reverse Genetics Approaches to Control Arenavirus.Methods Mol Biol. 2016;1403:313-51. doi: 10.1007/978-1-4939-3387-7_17. Methods Mol Biol. 2016. PMID: 27076139 Free PMC article.
-
Reverse genetics of rabies virus: new strategies to attenuate virus virulence for vaccine development.J Neurovirol. 2015 Aug;21(4):335-45. doi: 10.1007/s13365-015-0350-2. Epub 2015 May 21. J Neurovirol. 2015. PMID: 25994916
References
-
- Albertini, A. A., A. K. Wernimont, T. Muziol, R. B. Ravelli, C. R. Clapier, G. Schoehn, W. Weissenhorn, and R. W. Ruigrok. 2006. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science 313360-363. - PubMed
-
- Bedard, K. M., and B. L. Semler. 2004. Regulation of picornavirus gene expression. Microbes Infect. 6702-713. - PubMed
-
- Brinks, H., S. Conrad, J. Vogt, J. Oldekamp, A. Sierra, L. Deitinghoff, I. Bechmann, G. Alvarez-Bolado, B. Heimrich, P. P. Monnier, B. K. Mueller, and T. Skutella. 2004. The repulsive guidance molecule RGMa is involved in the formation of afferent connections in the dentate gyrus. J. Neurosci. 243862-3869. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

