Role for CCR5 in dissemination of vaccinia virus in vivo
- PMID: 19073715
- PMCID: PMC2643720
- DOI: 10.1128/JVI.01655-08
Role for CCR5 in dissemination of vaccinia virus in vivo
Abstract
In an earlier report, we provided evidence that expression of CCR5 by primary human T cells renders them permissive for vaccinia virus (VACV) replication. This may represent a mechanism for dissemination throughout the lymphatic system. To test this hypothesis, wild-type CCR5(+/+) and CCR5 null mice were challenged with VACV by intranasal inoculation. In time course studies using different infective doses of VACV, we identified viral replication in the lungs of both CCR5(+/+) and CCR5(-/-) mice, yet there were diminished viral loads in the spleens and brains of CCR5(-/-) mice compared with CCR5(+/+) mice. Moreover, in association with VACV infection, we provide evidence for CD4+ and CD8+ T-cell as well as CD11c+ and F4/80+ cell infiltration into the lungs of CCR5(+/+) but not CCR5(-/-) mice, and we show that the CCR5-expressing T cells harbor virus. We demonstrate that this CCR5 dependence is VACV specific, since CCR5(-/-) mice are as susceptible to intranasal influenza virus (A/WSN/33) infection as CCR5(+/+) mice. In a final series of experiments, we provide evidence that adoptive transfer of CCR5(+/+) bone marrow leukocytes into CCR5(-/-) mice restores VACV permissiveness, with evidence of lung and spleen infection. Taken together, our data suggest a novel role for CCR5 in VACV dissemination in vivo.
Figures
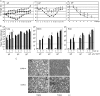
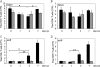
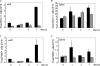
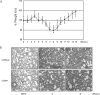
 ) mice (n = 10) at the indicated times p.i. with 104 PFU of VACV, as described in Materials and Methods.(C) Lungs of mock-infected or VACV-infected (104 PFU) CCR5+/+ and CCR5−/− mice were harvested on day 7 p.i., fixed in 2% paraformaldehyde, and embedded in paraffin, and 6-μm-thick histological sections were prepared and stained with hematoxylin-eosin. The dotted line indicates the lower level of detection for VACV. *, P < 0.05. ND, not detected.
) mice (n = 10) at the indicated times p.i. with 104 PFU of VACV, as described in Materials and Methods.(C) Lungs of mock-infected or VACV-infected (104 PFU) CCR5+/+ and CCR5−/− mice were harvested on day 7 p.i., fixed in 2% paraformaldehyde, and embedded in paraffin, and 6-μm-thick histological sections were prepared and stained with hematoxylin-eosin. The dotted line indicates the lower level of detection for VACV. *, P < 0.05. ND, not detected.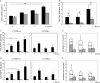
Similar articles
-
Both CD8+ and CD4+ T Cells Contribute to Corneal Clouding and Viral Clearance following Vaccinia Virus Infection in C57BL/6 Mice.J Virol. 2016 Jun 24;90(14):6557-6572. doi: 10.1128/JVI.00570-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27170749 Free PMC article.
-
Adverse events post smallpox-vaccination: insights from tail scarification infection in mice with Vaccinia virus.PLoS One. 2011 Apr 15;6(4):e18924. doi: 10.1371/journal.pone.0018924. PLoS One. 2011. PMID: 21526210 Free PMC article.
-
CD4(+) T-cell dependence of primary CD8(+) T-cell response against vaccinia virus depends upon route of infection and viral dose.Cell Mol Immunol. 2016 Jan;13(1):82-93. doi: 10.1038/cmi.2014.128. Epub 2014 Dec 29. Cell Mol Immunol. 2016. PMID: 25544501 Free PMC article.
-
Definition of epitopes and antigens recognized by vaccinia specific immune responses: their conservation in variola virus sequences, and use as a model system to study complex pathogens.Vaccine. 2009 Dec 30;27 Suppl 6(Suppl 6):G21-6. doi: 10.1016/j.vaccine.2009.10.011. Vaccine. 2009. PMID: 20006135 Free PMC article. Review.
-
Vaccinia Virus Protein C6: A Multifunctional Interferon Antagonist.Adv Exp Med Biol. 2018;1052:1-7. doi: 10.1007/978-981-10-7572-8_1. Adv Exp Med Biol. 2018. PMID: 29785476 Review.
Cited by
-
Downregulation of CCR5 expression on the peripheral blood CD8+ T cells of southeastern Iranian patients with chronic hepatitis B infection.Inflammation. 2013 Feb;36(1):136-40. doi: 10.1007/s10753-012-9528-4. Inflammation. 2013. PMID: 22918850
-
Chemokine binding protein vCCI attenuates vaccinia virus without affecting the cellular response elicited by immunization with a recombinant vaccinia vector carrying the HPV16 E7 gene.Viral Immunol. 2012 Oct;25(5):411-22. doi: 10.1089/vim.2011.0090. Viral Immunol. 2012. PMID: 23035852 Free PMC article.
-
Primary human macrophages serve as vehicles for vaccinia virus replication and dissemination.J Virol. 2014 Jun;88(12):6819-31. doi: 10.1128/JVI.03726-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696488 Free PMC article.
-
CCR5 on the NK Cells and its Ligand (RANTES) Expressions are Disrupted in South-Eastern Iranian Patients With Chronic Hepatitis B Infection.Iran Red Crescent Med J. 2014 Apr;16(4):e12458. doi: 10.5812/ircmj.12458. Epub 2014 Apr 5. Iran Red Crescent Med J. 2014. PMID: 24910790 Free PMC article.
-
Metabolomics as an Approach to Characterise the Contrasting Roles of CCR5 in the Presence and Absence of Disease.Int J Mol Sci. 2020 Feb 21;21(4):1472. doi: 10.3390/ijms21041472. Int J Mol Sci. 2020. PMID: 32098198 Free PMC article. Review.
References
-
- Alcami, A., J. A. Symons, P. D. Collins, T. J. Williams, and G. L. Smith. 1998. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 160624-633. - PubMed
-
- Andres, P. G., P. L. Beck, E. Mizoguchi, A. Mizoguchi, A. K. Bhan, T. Dawson, W. A. Kuziel, N. Maeda, R. P. MacDermott, D. K. Podolsky, and H. C. Reinecker. 2000. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol. 1646303-6312. - PubMed
-
- Baird, A. M., R. M. Gerstein, and L. J. Berg. 1999. The role of cytokine receptor signaling in lymphocyte development. Curr. Opin. Immunol. 11157-166. - PubMed
-
- Bartlett, N. W., K. Buttigieg, S. V. Kotenko, and G. L. Smith. 2005. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J. Gen. Virol. 861589-1596. - PubMed
-
- Breman, J. G., and D. A. Henderson. 2002. Diagnosis and management of smallpox. N. Engl. J. Med. 3461300-1308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

