How mitochondria produce reactive oxygen species
- PMID: 19061483
- PMCID: PMC2605959
- DOI: 10.1042/BJ20081386
How mitochondria produce reactive oxygen species
Abstract
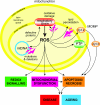
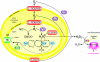
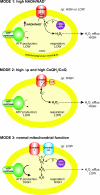
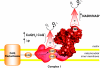
The production of ROS (reactive oxygen species) by mammalian mitochondria is important because it underlies oxidative damage in many pathologies and contributes to retrograde redox signalling from the organelle to the cytosol and nucleus. Superoxide (O2(*-)) is the proximal mitochondrial ROS, and in the present review I outline the principles that govern O2(*-) production within the matrix of mammalian mitochondria. The flux of O2(*-) is related to the concentration of potential electron donors, the local concentration of O2 and the second-order rate constants for the reactions between them. Two modes of operation by isolated mitochondria result in significant O2(*-) production, predominantly from complex I: (i) when the mitochondria are not making ATP and consequently have a high Deltap (protonmotive force) and a reduced CoQ (coenzyme Q) pool; and (ii) when there is a high NADH/NAD+ ratio in the mitochondrial matrix. For mitochondria that are actively making ATP, and consequently have a lower Deltap and NADH/NAD+ ratio, the extent of O2(*-) production is far lower. The generation of O2(*-) within the mitochondrial matrix depends critically on Deltap, the NADH/NAD+ and CoQH2/CoQ ratios and the local O2 concentration, which are all highly variable and difficult to measure in vivo. Consequently, it is not possible to estimate O2(*-) generation by mitochondria in vivo from O2(*-)-production rates by isolated mitochondria, and such extrapolations in the literature are misleading. Even so, the description outlined here facilitates the understanding of factors that favour mitochondrial ROS production. There is a clear need to develop better methods to measure mitochondrial O2(*-) and H2O2 formation in vivo, as uncertainty about these values hampers studies on the role of mitochondrial ROS in pathological oxidative damage and redox signalling.
Figures

Similar articles
-
Control of mitochondrial superoxide production by reverse electron transport at complex I.J Biol Chem. 2018 Jun 22;293(25):9869-9879. doi: 10.1074/jbc.RA118.003647. Epub 2018 May 9. J Biol Chem. 2018. PMID: 29743240 Free PMC article.
-
Production of reactive oxygen species by complex I (NADH:ubiquinone oxidoreductase) from Escherichia coli and comparison to the enzyme from mitochondria.Biochemistry. 2008 Mar 25;47(12):3964-71. doi: 10.1021/bi702243b. Epub 2008 Feb 29. Biochemistry. 2008. PMID: 18307315
-
Identifying Site-Specific Superoxide and Hydrogen Peroxide Production Rates From the Mitochondrial Electron Transport System Using a Computational Strategy.Function (Oxf). 2021 Sep 20;2(6):zqab050. doi: 10.1093/function/zqab050. eCollection 2021. Function (Oxf). 2021. PMID: 35330793 Free PMC article.
-
Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species.Redox Biol. 2015;4:381-98. doi: 10.1016/j.redox.2015.02.001. Epub 2015 Feb 7. Redox Biol. 2015. PMID: 25744690 Free PMC article. Review.
-
Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling.Free Radic Biol Med. 2016 Nov;100:14-31. doi: 10.1016/j.freeradbiomed.2016.04.001. Epub 2016 Apr 13. Free Radic Biol Med. 2016. PMID: 27085844 Review.
Cited by
-
Structure of the turnover-ready state of an ancestral respiratory complex I.Nat Commun. 2024 Oct 29;15(1):9340. doi: 10.1038/s41467-024-53679-3. Nat Commun. 2024. PMID: 39472559 Free PMC article.
-
Physiological and Genetic Basis of High-Altitude Indigenous Animals' Adaptation to Hypoxic Environments.Animals (Basel). 2024 Oct 19;14(20):3031. doi: 10.3390/ani14203031. Animals (Basel). 2024. PMID: 39457960 Free PMC article. Review.
-
Frequency-Dependent Antioxidant Responses in HT-1080 Human Fibrosarcoma Cells Exposed to Weak Radio Frequency Fields.Antioxidants (Basel). 2024 Oct 15;13(10):1237. doi: 10.3390/antiox13101237. Antioxidants (Basel). 2024. PMID: 39456490 Free PMC article.
-
PET Imaging with [18F]ROStrace Detects Oxidative Stress and Predicts Parkinson's Disease Progression in Mice.Antioxidants (Basel). 2024 Oct 12;13(10):1226. doi: 10.3390/antiox13101226. Antioxidants (Basel). 2024. PMID: 39456479 Free PMC article.
References
-
- Andreyev A. Y., Kushnareva Y. E., Starkov A. A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Moscow) 2005;70:200–214. - PubMed
-
- Balaban R. S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
-
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. - PubMed
-
- Cadenas E., Davies K. J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biol. Med. 2000;29:222–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

