A conserved domain in the leader proteinase of foot-and-mouth disease virus is required for proper subcellular localization and function
- PMID: 19052079
- PMCID: PMC2643771
- DOI: 10.1128/JVI.02112-08
A conserved domain in the leader proteinase of foot-and-mouth disease virus is required for proper subcellular localization and function
Abstract
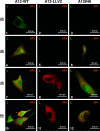
The leader proteinase (L(pro)) of foot-and-mouth disease virus (FMDV) is involved in antagonizing the innate immune response by blocking the expression of interferon (IFN) and by reducing the immediate-early induction of IFN-beta mRNA and IFN-stimulated genes. In addition to its role in shutting off cap-dependent host mRNA translation, L(pro) is associated with the degradation of the p65/RelA subunit of nuclear factor kappaB (NF-kappaB). Bioinformatics analysis suggests that L(pro) contains a SAP (for SAF-A/B, Acinus, and PIAS) domain, a protein structure associated in some cases with the nuclear retention of molecules involved in transcriptional control. We have introduced a single or a double mutation in conserved amino acid residues contained within this domain of L(pro). Although three stable mutant viruses were obtained, only the double mutant displayed an attenuated phenotype in cell culture. Indirect immunofluorescence analysis showed that L(pro) subcellular distribution is altered in cells infected with the double mutant virus. Interestingly, nuclear p65/RelA staining disappeared from wild-type (WT) FMDV-infected cells but not from double mutant virus-infected cells. Consistent with these results, NF-kappaB-dependent transcription was not inhibited in cells infected with double mutant virus in contrast to cells infected with WT virus. However, degradation of the translation initiation factor eIF-4G was very similar for both the WT and the double mutant viruses. Since L(pro) catalytic activity was demonstrated to be a requirement for p65/RelA degradation, our results indicate that mutation of the SAP domain reveals a novel separation-of-function activity for FMDV L(pro).
Figures




Similar articles
-
Degradation of nuclear factor kappa B during foot-and-mouth disease virus infection.J Virol. 2007 Dec;81(23):12803-15. doi: 10.1128/JVI.01467-07. Epub 2007 Sep 19. J Virol. 2007. PMID: 17881445 Free PMC article.
-
Characterization of a chimeric foot-and-mouth disease virus bearing a bovine rhinitis B virus leader proteinase.Virology. 2013 Dec;447(1-2):172-80. doi: 10.1016/j.virol.2013.08.035. Epub 2013 Oct 1. Virology. 2013. PMID: 24210112
-
Foot-and-Mouth Disease Virus Antagonizes NOD2-Mediated Antiviral Effects by Inhibiting NOD2 Protein Expression.J Virol. 2019 May 15;93(11):e00124-19. doi: 10.1128/JVI.00124-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894473 Free PMC article.
-
Multifunctional roles of leader protein of foot-and-mouth disease viruses in suppressing host antiviral responses.Vet Res. 2015 Oct 28;46:127. doi: 10.1186/s13567-015-0273-1. Vet Res. 2015. PMID: 26511922 Free PMC article. Review.
-
Evading the host immune response: how foot-and-mouth disease virus has become an effective pathogen.FEMS Immunol Med Microbiol. 2008 Jun;53(1):8-17. doi: 10.1111/j.1574-695X.2008.00409.x. Epub 2008 Apr 8. FEMS Immunol Med Microbiol. 2008. PMID: 18400012 Review.
Cited by
-
Differential gene expression in porcine SK6 cells infected with wild-type and SAP domain-mutant foot-and-mouth disease virus.Virol Sin. 2016 Jun;31(3):249-57. doi: 10.1007/s12250-015-3709-x. Epub 2016 Apr 8. Virol Sin. 2016. PMID: 27097918 Free PMC article.
-
Pathogenesis of virulent and attenuated foot-and-mouth disease virus in cattle.Virol J. 2017 May 2;14(1):89. doi: 10.1186/s12985-017-0758-9. Virol J. 2017. PMID: 28464897 Free PMC article.
-
Innate Immune Detection of Cardioviruses and Viral Disruption of Interferon Signaling.Front Microbiol. 2018 Oct 12;9:2448. doi: 10.3389/fmicb.2018.02448. eCollection 2018. Front Microbiol. 2018. PMID: 30369921 Free PMC article. Review.
-
Structure and Function of Viral Deubiquitinating Enzymes.J Mol Biol. 2017 Nov 10;429(22):3441-3470. doi: 10.1016/j.jmb.2017.06.010. Epub 2017 Jun 16. J Mol Biol. 2017. PMID: 28625850 Free PMC article. Review.
-
Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling.J Virol. 2012 Sep;86(17):9311-22. doi: 10.1128/JVI.00722-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718831 Free PMC article.
References
-
- Aravind, L., and E. V. Koonin. 2000. SAP: a putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 25112-114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

