Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes
- PMID: 19050264
- PMCID: PMC2665797
- DOI: 10.4049/jimmunol.181.12.8460
Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes
Abstract
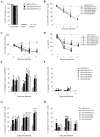
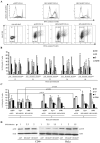
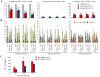
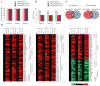
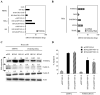
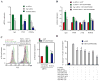
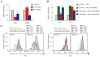
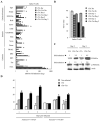
The high mutation rate of HIV is linked to the generation of viruses expressing proteins with altered function whose impact on disease progression is unknown. We investigated how HIV-1 viruses lacking Env, Vpr, and Nef affect CD4(+) T cell survival. We found that in the absence of these proteins, HIV-1-infected CD4(+) primary T cells progress to the G(0) phase of the cell cycle and to cell death, indicating that viruses expressing inactive forms of these proteins can contribute to the CD4(+) T cell decline as the wild-type virus, suggesting that other HIV proteins are responsible for inducing apoptosis. Apoptosis in these cells is triggered by the alteration of the Egr1-PTEN-Akt (early growth response-1/phosphate and tensin homolog deleted on chromosome 10/Akt) and p53 pathways, which converge on the FOXO3a (Forkhead box transcription factor O class 3a) transcriptional activator. The FOXO3a target genes Fas ligand and TRAIL, involved in the extrinsic apoptotic pathway, and PUMA, Noxa, and Bim, which are part of the intrinsic apoptotic pathway, were also up-regulated, indicating that HIV infection leads to apoptosis by the engagement of multiple apoptotic pathways. RNAi-mediated knockdown of Egr1 and FOXO3a resulted in reduced apoptosis in HIV-infected HeLa and CD4(+) T cells, providing further evidence for their critical role in HIV-induced apoptosis and G(0) arrest. We tested the possibility that Tat is responsible for the T cell apoptosis observed with these mutant viruses. The induction of Egr1 and FOXO3a and its target genes was observed in Jurkat cells transduced by Tat alone. Tat-dependent activation of the Egr1-PTEN-FOXO3a pathway provides a mechanism for HIV-1-associated CD4(+) T cell death.
Figures

Similar articles
-
Association of Tat with promoters of PTEN and PP2A subunits is key to transcriptional activation of apoptotic pathways in HIV-infected CD4+ T cells.PLoS Pathog. 2010 Sep 16;6(9):e1001103. doi: 10.1371/journal.ppat.1001103. PLoS Pathog. 2010. PMID: 20862322 Free PMC article.
-
Modulation of apoptosis and viral latency - an axis to be well understood for successful cure of human immunodeficiency virus.J Gen Virol. 2016 Apr;97(4):813-824. doi: 10.1099/jgv.0.000402. Epub 2016 Jan 13. J Gen Virol. 2016. PMID: 26764023 Review.
-
Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence.J Immunol. 2000 Dec 1;165(11):6437-46. doi: 10.4049/jimmunol.165.11.6437. J Immunol. 2000. PMID: 11086083
-
Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4+ T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-kappaB, and AP-1 induction.J Biol Chem. 2004 Sep 17;279(38):39520-31. doi: 10.1074/jbc.M407477200. Epub 2004 Jul 16. J Biol Chem. 2004. PMID: 15258149
-
Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells?Biochem Biophys Res Commun. 2000 Jan 27;267(3):677-85. doi: 10.1006/bbrc.1999.1708. Biochem Biophys Res Commun. 2000. PMID: 10673351 Review.
Cited by
-
Nature and nurture: T-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool.Immunology. 2010 Nov;131(3):310-7. doi: 10.1111/j.1365-2567.2010.03338.x. Epub 2010 Aug 25. Immunology. 2010. PMID: 20738422 Free PMC article. Review.
-
DRAM triggers lysosomal membrane permeabilization and cell death in CD4(+) T cells infected with HIV.PLoS Pathog. 2013;9(5):e1003328. doi: 10.1371/journal.ppat.1003328. Epub 2013 May 2. PLoS Pathog. 2013. PMID: 23658518 Free PMC article. Clinical Trial.
-
In-Vitro Subtype-Specific Modulation of HIV-1 Trans-Activator of Transcription (Tat) on RNAi Silencing Suppressor Activity and Cell Death.Viruses. 2019 Oct 23;11(11):976. doi: 10.3390/v11110976. Viruses. 2019. PMID: 31652847 Free PMC article.
-
CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis.Viruses. 2011 May;3(5):586-612. doi: 10.3390/v3050586. Epub 2011 May 12. Viruses. 2011. PMID: 21994747 Free PMC article. Review.
-
Pathogens and Carcinogenesis: A Review.Biology (Basel). 2021 Jun 15;10(6):533. doi: 10.3390/biology10060533. Biology (Basel). 2021. PMID: 34203649 Free PMC article. Review.
References
-
- Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med. 2007;58:461–476. - PubMed
-
- Cotton MF, Ikle DN, Rapaport EL, Marschner S, Tseng PO, Kurrle R, Finkel TH. Apoptosis of CD4+ and CD8+ T cells isolated immediately ex vivo correlates with disease severity in human immunodeficiency virus type 1 infection. Pediatr Res. 1997;42:656–664. - PubMed
-
- Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. - PubMed
-
- Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

