Highly dissociative and concerted mechanism for the nicotinamide cleavage reaction in Sir2Tm enzyme suggested by ab initio QM/MM molecular dynamics simulations
- PMID: 19049465
- PMCID: PMC2627508
- DOI: 10.1021/ja807269j
Highly dissociative and concerted mechanism for the nicotinamide cleavage reaction in Sir2Tm enzyme suggested by ab initio QM/MM molecular dynamics simulations
Abstract
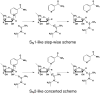
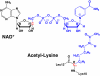
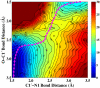
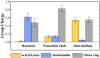
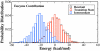
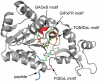
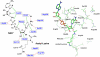
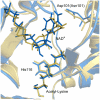
Sir2 enzymes catalyze the NAD+-dependent protein deacetylation and play critical roles in epigenetics, cell death, and lifespan regulation. In spite of a current flurry of experimental studies, the catalytic mechanism for this unique and important class of enzymes remains elusive. Employing on-the-fly Born-Oppenheimer molecular dynamics simulations with the B3LYP/6-31G(d) QM/MM potential and the umbrella sampling method, we have characterized the initial step of the Sir2Tm-catalyzed reaction, which is also the most controversial portion of its mechanism. Our results indicate that the nicotinamide cleavage reaction employs a highly dissociative and concerted displacement mechanism: the cleavage of the glycosidic bond is facilitated by the nucleophilic participation of the acetyl-lysine, and the dissociative transition state has a significant oxocarbenium ion character. During this step of the reaction, the Sir2Tm enzyme strongly stabilizes the covalent O-alkylamidate intermediate whereas its effect on the transition state is quite minimal. In addition, functional roles of key residues and motifs have been elucidated. This work further demonstrates the feasibility and applicability of the state-of-the-art ab initio QM/MM molecular dynamics approach in simulating enzyme reactions.
Figures


Similar articles
-
Catalytic reaction mechanism of acetylcholinesterase determined by Born-Oppenheimer ab initio QM/MM molecular dynamics simulations.J Phys Chem B. 2010 Jul 8;114(26):8817-25. doi: 10.1021/jp104258d. J Phys Chem B. 2010. PMID: 20550161 Free PMC article.
-
Investigation of the catalytic mechanism of Sir2 enzyme with QM/MM approach: SN1 vs SN2?J Phys Chem B. 2010 Sep 16;114(36):11927-33. doi: 10.1021/jp1054183. J Phys Chem B. 2010. PMID: 20726530
-
Structural insights into intermediate steps in the Sir2 deacetylation reaction.Structure. 2008 Sep 10;16(9):1368-77. doi: 10.1016/j.str.2008.05.015. Structure. 2008. PMID: 18786399 Free PMC article.
-
SIR2: the biochemical mechanism of NAD(+)-dependent protein deacetylation and ADP-ribosyl enzyme intermediates.Curr Med Chem. 2004 Apr;11(7):807-26. doi: 10.2174/0929867043455675. Curr Med Chem. 2004. PMID: 15078167 Review.
-
Mechanism-based modulator discovery for sirtuin-catalyzed deacetylation reaction.Mini Rev Med Chem. 2013 Jan;13(1):132-54. Mini Rev Med Chem. 2013. PMID: 22876953 Review.
Cited by
-
Born-Oppenheimer ab initio QM/MM molecular dynamics simulations of the hydrolysis reaction catalyzed by protein arginine deiminase 4.J Phys Chem B. 2009 Dec 31;113(52):16705-10. doi: 10.1021/jp9080614. J Phys Chem B. 2009. PMID: 20028143 Free PMC article.
-
Solvation Free Energy Calculations with Quantum Mechanics/Molecular Mechanics and Machine Learning Models.J Phys Chem B. 2019 Jan 31;123(4):901-908. doi: 10.1021/acs.jpcb.8b11905. Epub 2019 Jan 15. J Phys Chem B. 2019. PMID: 30557020 Free PMC article.
-
Intrinsic Cleavage of RNA Polymerase II Adopts a Nucleobase-independent Mechanism Assisted by Transcript Phosphate.Nat Energy. 2019 Mar;2(3):228-235. doi: 10.1038/s41929-019-0227-5. Epub 2019 Feb 11. Nat Energy. 2019. PMID: 31179024 Free PMC article.
-
Insights into the phosphoryl transfer mechanism of cyclin-dependent protein kinases from ab initio QM/MM free-energy studies.J Phys Chem B. 2011 Nov 24;115(46):13713-22. doi: 10.1021/jp207532s. Epub 2011 Nov 3. J Phys Chem B. 2011. PMID: 21999515 Free PMC article.
-
Ab initio QM/MM free-energy studies of arginine deiminase catalysis: the protonation state of the Cys nucleophile.J Phys Chem B. 2011 Apr 7;115(13):3725-33. doi: 10.1021/jp200843s. Epub 2011 Mar 11. J Phys Chem B. 2011. PMID: 21395290 Free PMC article.
References
-
- Blander G, Guarente L. Annu. Rev. Biochem. 2004;73:417–435. - PubMed
-
- Marmorstein R. Biochem. Soc. Trans. 2004;32:904–909. - PubMed
-
- Denu JM. Curr. Opin. Chem. biol. 2005;9:431–440. - PubMed
-
- Sauve AA, Wolberger C, Schramm VL, Boeke JD. Annu. Rev. Biochem. 2006;75:435–465. - PubMed
-
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Genes Dev. 1993;7:592–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

