The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing
- PMID: 19042970
- PMCID: PMC2647315
- DOI: 10.1093/nar/gkn937
The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing
Abstract
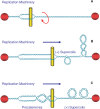
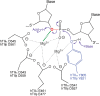
Topoisomerase II is an essential enzyme that is required for virtually every process that requires movement of DNA within the nucleus or the opening of the double helix. This enzyme helps to regulate DNA under- and overwinding and removes knots and tangles from the genetic material. In order to carry out its critical physiological functions, topoisomerase II generates transient double-stranded breaks in DNA. Consequently, while necessary for cell survival, the enzyme also has the capacity to fragment the genome. The DNA cleavage/ligation reaction of topoisomerase II is the target for some of the most successful anticancer drugs currently in clinical use. However, this same reaction also is believed to trigger chromosomal translocations that are associated with specific types of leukemia. This article will familiarize the reader with the DNA cleavage/ligation reaction of topoisomerase II and other aspects of its catalytic cycle. In addition, it will discuss the interaction of the enzyme with anticancer drugs and the mechanisms by which these agents increase levels of topoisomerase II-generated DNA strand breaks. Finally, it will describe dietary and environmental agents that enhance DNA cleavage mediated by the enzyme.
Figures


Similar articles
-
Stabilization of eukaryotic topoisomerase II-DNA cleavage complexes.Curr Top Med Chem. 2003;3(3):321-38. doi: 10.2174/1568026033452519. Curr Top Med Chem. 2003. PMID: 12570766 Review.
-
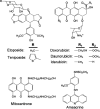
Etoposide, topoisomerase II and cancer.Curr Med Chem Anticancer Agents. 2005 Jul;5(4):363-72. doi: 10.2174/1568011054222364. Curr Med Chem Anticancer Agents. 2005. PMID: 16101488 Review.
-
Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice.Prog Nucleic Acid Res Mol Biol. 2000;64:221-53. doi: 10.1016/s0079-6603(00)64006-0. Prog Nucleic Acid Res Mol Biol. 2000. PMID: 10697411 Review.
-
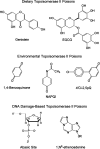
DNA topoisomerase II, genotoxicity, and cancer.Mutat Res. 2007 Oct 1;623(1-2):83-97. doi: 10.1016/j.mrfmmm.2007.06.009. Epub 2007 Jul 3. Mutat Res. 2007. PMID: 17681352 Free PMC article. Review.
-
Topoisomerase II Poisons: Converting Essential Enzymes into Molecular Scissors.Biochemistry. 2021 Jun 1;60(21):1630-1641. doi: 10.1021/acs.biochem.1c00240. Epub 2021 May 19. Biochemistry. 2021. PMID: 34008964 Free PMC article. Review.
Cited by
-
Unraveling curcumin degradation: autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione.J Biol Chem. 2015 Feb 20;290(8):4817-4828. doi: 10.1074/jbc.M114.618785. Epub 2015 Jan 6. J Biol Chem. 2015. PMID: 25564617 Free PMC article.
-
DNA binding activity of the proximal C-terminal domain of rat DNA topoisomerase IIβ is involved in ICRF-193-induced closed-clamp formation.PLoS One. 2020 Sep 22;15(9):e0239466. doi: 10.1371/journal.pone.0239466. eCollection 2020. PLoS One. 2020. PMID: 32960919 Free PMC article.
-
Efficacy of substituted 9-aminoacridine derivatives in small cell lung cancer.Invest New Drugs. 2013 Apr;31(2):285-92. doi: 10.1007/s10637-012-9854-2. Epub 2012 Jul 22. Invest New Drugs. 2013. PMID: 22821172
-
Combined PARP and Dual Topoisomerase Inhibition Potentiates Genome Instability and Cell Death in Ovarian Cancer.Int J Mol Sci. 2022 Sep 10;23(18):10503. doi: 10.3390/ijms231810503. Int J Mol Sci. 2022. PMID: 36142413 Free PMC article.
-
Synthesis and molecular docking studies of some novel Schiff bases incorporating 6-butylquinolinedione moiety as potential topoisomerase IIβ inhibitors.R Soc Open Sci. 2018 Jun 20;5(6):172407. doi: 10.1098/rsos.172407. eCollection 2018 Jun. R Soc Open Sci. 2018. PMID: 30110445 Free PMC article.
References
-
- Loeb LA, Monnat R.J., Jr. DNA polymerases and human disease. Nat. Rev. Genet. 2008;9:594–604. - PubMed
-
- Baguley BC, Ferguson LR. Mutagenic properties of topoisomerase-targeted drugs. Biochim. Biophys. Acta. 1998;1400:213–222. - PubMed
-
- Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog. Nucleic Acid Res. Mol. Biol. 2000;64:221–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

