Functional characterization of a small heat shock protein from Mycobacterium leprae
- PMID: 19040732
- PMCID: PMC2629775
- DOI: 10.1186/1471-2180-8-208
Functional characterization of a small heat shock protein from Mycobacterium leprae
Abstract
Background: Small heat shock proteins are ubiquitous family of stress proteins, having a role in virulence and survival of the pathogen. M. leprae, the causative agent of leprosy is an uncultivable organism in defined media, hence the biology and function of proteins were examined by cloning M. leprae genes in heterologous hosts. The study on sHsp18 was carried out as the knowledge about the functions of this major immunodominant antigen of M. leprae is scanty.
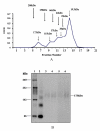
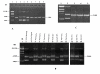
Results: The gene encoding Mycobacterium leprae small heat shock protein (sHsp18) was amplified from biopsy material of leprosy patients, and cloned and expressed in E. coli. The localization and in vitro characterization of the protein are detailed in this report. Data show that major portion of the protein is localized in the outer membrane of E. coli. The purified sHsp18 functions as an efficient chaperone as shown by their ability to prevent thermal inactivation of restriction enzymes SmaI and NdeI. Physical interaction of the chaperone with target protein is also demonstrated. Size exclusion chromatography of purified protein shows that the protein can form multimeric complexes under in vitro conditions as is demonstrated for several small heat shock proteins.
Conclusion: The small heat shock protein sHsp18 of M. leprae is a chaperone and shows several properties associated with other small heat shock proteins. Membrane association and in vitro chaperone function of sHsp18 shows that the protein may play a role in the virulence and survival of M. leprae in infected host.
Figures

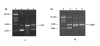
Similar articles
-
Protective role of Mycobacterium leprae small heat-shock protein in heterologous hosts, Escherichia coli and Mycobacterium smegmatis, grown under stress.J Med Microbiol. 2013 Jul;62(Pt 7):959-967. doi: 10.1099/jmm.0.057851-0. Epub 2013 Apr 11. J Med Microbiol. 2013. PMID: 23579398
-
Structure, stability and chaperone function of Mycobacterium leprae Heat Shock Protein 18 are differentially affected upon interaction with gold and silver nanoparticles.Int J Biol Macromol. 2020 Jun 1;152:250-260. doi: 10.1016/j.ijbiomac.2020.02.182. Epub 2020 Feb 19. Int J Biol Macromol. 2020. PMID: 32084461
-
Interaction of constituents of MDT regimen for leprosy with Mycobacterium leprae HSP18: impact on its structure and function.FEBS J. 2022 Feb;289(3):832-853. doi: 10.1111/febs.16212. Epub 2021 Oct 10. FEBS J. 2022. PMID: 34555271
-
Multifaceted role of lipids in Mycobacterium leprae.Future Microbiol. 2017 Mar;12:315-335. doi: 10.2217/fmb-2016-0173. Epub 2017 Mar 13. Future Microbiol. 2017. PMID: 28287297 Review.
-
The microbiology of Mycobacterium leprae, Part II. Reflections on major developments and those responsible for them.Int J Lepr Other Mycobact Dis. 1994 Dec;62(4):594-8. Int J Lepr Other Mycobact Dis. 1994. PMID: 7868959 Review. No abstract available.
Cited by
-
Characterization of rice small heat shock proteins targeted to different cellular organelles.Cell Stress Chaperones. 2015 May;20(3):451-60. doi: 10.1007/s12192-015-0570-7. Epub 2015 Jan 28. Cell Stress Chaperones. 2015. PMID: 25624002 Free PMC article.
-
Small but mighty: a functional look at bacterial sHSPs.Cell Stress Chaperones. 2020 Jul;25(4):593-600. doi: 10.1007/s12192-020-01094-0. Epub 2020 Apr 16. Cell Stress Chaperones. 2020. PMID: 32301005 Free PMC article. Review.
-
Small heat-shock protein HspL is induced by VirB protein(s) and promotes VirB/D4-mediated DNA transfer in Agrobacterium tumefaciens.Microbiology (Reading). 2009 Oct;155(Pt 10):3270-3280. doi: 10.1099/mic.0.030676-0. Epub 2009 Jun 25. Microbiology (Reading). 2009. PMID: 19556291 Free PMC article.
-
Role of ATP-Small Heat Shock Protein Interaction in Human Diseases.Front Mol Biosci. 2022 Feb 16;9:844826. doi: 10.3389/fmolb.2022.844826. eCollection 2022. Front Mol Biosci. 2022. PMID: 35252358 Free PMC article. Review.
-
Interaction of ATP with a small heat shock protein from Mycobacterium leprae: effect on its structure and function.PLoS Negl Trop Dis. 2015 Mar 26;9(3):e0003661. doi: 10.1371/journal.pntd.0003661. eCollection 2015 Mar. PLoS Negl Trop Dis. 2015. PMID: 25811190 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

