Study of oncogenic transformation in ex vivo expanded mesenchymal cells, from paediatric bone marrow
- PMID: 19040569
- PMCID: PMC6496314
- DOI: 10.1111/j.1365-2184.2008.00559.x
Study of oncogenic transformation in ex vivo expanded mesenchymal cells, from paediatric bone marrow
Abstract
Objectives: Mesenchymal stromal cells (MSCs) have attracted considerable interest in both the scientific and clinical fields. In order to obtain a sufficient cell number for application, their in vitro expansion is necessary, but during this process their characteristics may be altered and cells may acquire oncogenic properties. We have investigated properties of MSC that may be related to oncogenesis, a critical parameter that has to be evaluated prior to MSC clinical use.
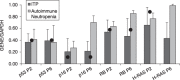
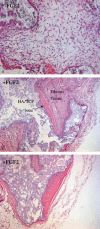
Materials and methods: We studied the expression of p53, p16, RB, H-RAS and human telomerase reverse transcriptase (hTERT) in MSCs from bone marrow of children diagnosed with idiopathic thrombocytopenic purpura (ITP) and autoimmune neutropenia. The same cells were seeded in soft agar to confirm their anchorage dependence and were karyotypically analysed. Finally, MSCs were subcutaneously transplanted into SCID mice and their ectopic osteogenic as well as tumorigenic potential was evaluated.
Results: We have shown that MSCs derived from bone marrow of children with ITP and autoimmune neutropenia do not undergo transformation, the cells expressed normal levels of p53, p16, RB and H-RAS. Expression of hTERT was undetectable, chromosome content remained stable, and their anchorage dependence was confirmed. In an in vivo model, when MSCs were subcutaneously transplanted into SCID mice, no tumorigenesis was observed.
Conclusions: These findings suggest that MSCs from bone marrow of children do not have oncogenic properties and, therefore, represent validate candidates for applications in regenerative medicine.
Figures






Similar articles
-
Modeling sarcomagenesis using multipotent mesenchymal stem cells.Cell Res. 2012 Jan;22(1):62-77. doi: 10.1038/cr.2011.157. Epub 2011 Sep 20. Cell Res. 2012. PMID: 21931359 Free PMC article. Review.
-
Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms.Cancer Res. 2007 Oct 1;67(19):9142-9. doi: 10.1158/0008-5472.CAN-06-4690. Cancer Res. 2007. PMID: 17909019
-
Deficiency in p53 but not retinoblastoma induces the transformation of mesenchymal stem cells in vitro and initiates leiomyosarcoma in vivo.Cancer Res. 2010 May 15;70(10):4185-94. doi: 10.1158/0008-5472.CAN-09-4640. Epub 2010 May 4. Cancer Res. 2010. PMID: 20442289
-
Neoplastic transformation by TERT in FGF-2-expanded human mesenchymal stem cells.Int J Oncol. 2011 Jul;39(1):5-11. doi: 10.3892/ijo.2011.1029. Epub 2011 May 3. Int J Oncol. 2011. PMID: 21573488
-
Improving cell therapy--experiments using transplanted telomerase-immortalized cells in immunodeficient mice.Mech Ageing Dev. 2007 Jan;128(1):25-30. doi: 10.1016/j.mad.2006.11.006. Epub 2006 Nov 22. Mech Ageing Dev. 2007. PMID: 17123586 Free PMC article. Review.
Cited by
-
Modeling sarcomagenesis using multipotent mesenchymal stem cells.Cell Res. 2012 Jan;22(1):62-77. doi: 10.1038/cr.2011.157. Epub 2011 Sep 20. Cell Res. 2012. PMID: 21931359 Free PMC article. Review.
-
A shorter telomere is the key factor in preventing cultured human mesenchymal stem cells from senescence escape.Histochem Cell Biol. 2014 Sep;142(3):257-67. doi: 10.1007/s00418-014-1210-5. Histochem Cell Biol. 2014. PMID: 24658836
-
The potential of mesenchymal stem cells in the management of radiation enteropathy.Cell Death Dis. 2015 Aug 6;6(8):e1840. doi: 10.1038/cddis.2015.189. Cell Death Dis. 2015. PMID: 26247725 Free PMC article. Review.
-
A Comparison of Phenotypic and Functional Properties of Mesenchymal Stromal Cells and Multipotent Adult Progenitor Cells.Front Immunol. 2019 Aug 28;10:1952. doi: 10.3389/fimmu.2019.01952. eCollection 2019. Front Immunol. 2019. PMID: 31555259 Free PMC article. Review.
-
Sox11 is expressed in early progenitor human multipotent stromal cells and decreases with extensive expansion of the cells.Tissue Eng Part A. 2010 Nov;16(11):3385-94. doi: 10.1089/ten.tea.2010.0085. Epub 2010 Jul 13. Tissue Eng Part A. 2010. PMID: 20626275 Free PMC article.
References
-
- Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57, 874–882. - PubMed
-
- Barry FP (2003) Mesenchymal stem cell therapy in joint disease. Novartis Found. Symp. 249, 86–96. - PubMed
-
- Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F (2007) Human bone marrow derived mesenchymal stem cells do not undergo transformation after long‐term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 67, 9142–9149. - PubMed
-
- Campagnolli C, Bellantuonno I, Kumar S, Fairnairn LJ, Roberts I, Fisk NM (2002) High transduction efficiency of circulating first trimester fetal mesenchymal cells: potential targets for in utero ex vivo gene therapy. BJOG 109, 952–954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

