Leucine-rich repeat kinase 2 (LRRK2): a key player in the pathogenesis of Parkinson's disease
- PMID: 19025767
- PMCID: PMC4072732
- DOI: 10.1002/jnr.21949
Leucine-rich repeat kinase 2 (LRRK2): a key player in the pathogenesis of Parkinson's disease
Abstract
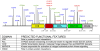
Parkinson's disease (PD) is the most common neurodegenerative movement disorder, with a prevalence of more than 1% after the age of 65 years. Mutations in the gene encoding leucine-rich repeat kinase-2 (LRRK2) have recently been linked to autosomal dominant, late-onset PD that is clinically indistinguishable from typical, idiopathic disease. LRRK2 is a multidomain protein containing several protein interaction motifs as well as dual enzymatic domains of GTPase and protein kinase activities. Disease-associated mutations are found throughout the multidomain structure of the protein. LRRK2, however, is unique among the PD-causing genes, because a missense mutation, G2019S, is a frequent determinant of not only familial but also sporadic PD. Thus, LRRK2 has emerged as a promising therapeutic target for combating PD. In this Mini-Review, we look at the current state of knowledge regarding the domain structure, amino acid substitutions, and potential functional roles of LRRK2.
Figures
Similar articles
-
LRRK2 in Parkinson's disease: protein domains and functional insights.Trends Neurosci. 2006 May;29(5):286-93. doi: 10.1016/j.tins.2006.03.006. Epub 2006 Apr 17. Trends Neurosci. 2006. PMID: 16616379 Review.
-
Biochemical and molecular features of LRRK2 and its pathophysiological roles in Parkinson's disease.BMB Rep. 2010 Apr;43(4):233-44. doi: 10.5483/bmbrep.2010.43.4.233. BMB Rep. 2010. PMID: 20423607 Review.
-
Contribution of GTPase activity to LRRK2-associated Parkinson disease.Small GTPases. 2013 Jul-Sep;4(3):164-70. doi: 10.4161/sgtp.25130. Epub 2013 Jun 10. Small GTPases. 2013. PMID: 24025585 Free PMC article. Review.
-
Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity.Proc Natl Acad Sci U S A. 2005 Nov 15;102(46):16842-7. doi: 10.1073/pnas.0507360102. Epub 2005 Nov 3. Proc Natl Acad Sci U S A. 2005. PMID: 16269541 Free PMC article.
-
The G2385R variant of leucine-rich repeat kinase 2 associated with Parkinson's disease is a partial loss-of-function mutation.Biochem J. 2012 Aug 15;446(1):99-111. doi: 10.1042/BJ20120637. Biochem J. 2012. PMID: 22612223 Free PMC article.
Cited by
-
Cellular processes associated with LRRK2 function and dysfunction.FEBS J. 2015 Aug;282(15):2806-26. doi: 10.1111/febs.13305. Epub 2015 May 9. FEBS J. 2015. PMID: 25899482 Free PMC article. Review.
-
Brain Penetrant LRRK2 Inhibitor.ACS Med Chem Lett. 2012 Aug 9;3(8):658-662. doi: 10.1021/ml300123a. Epub 2012 Jun 18. ACS Med Chem Lett. 2012. PMID: 23066449 Free PMC article.
-
Leucine-rich repeat kinase 2 modulates neuroinflammation and neurotoxicity in models of human immunodeficiency virus 1-associated neurocognitive disorders.J Neurosci. 2015 Apr 1;35(13):5271-83. doi: 10.1523/JNEUROSCI.0650-14.2015. J Neurosci. 2015. PMID: 25834052 Free PMC article.
-
Non-cell autonomous mechanism of Parkinson's disease pathology caused by G2019S LRRK2 mutation in Ashkenazi Jewish patient: Single cell analysis.Brain Res. 2019 Nov 1;1722:146342. doi: 10.1016/j.brainres.2019.146342. Epub 2019 Jul 19. Brain Res. 2019. PMID: 31330122 Free PMC article. Review.
-
Synthesis and Preliminary Evaluation of [11 C]GNE-1023 as a Potent PET Probe for Imaging Leucine-Rich Repeat Kinase 2 (LRRK2) in Parkinson's Disease.ChemMedChem. 2019 Sep 4;14(17):1580-1585. doi: 10.1002/cmdc.201900321. Epub 2019 Aug 22. ChemMedChem. 2019. PMID: 31365783 Free PMC article.
References
-
- Aasly JO, Toft M, Fernandez-Mata I, Kachergus J, Hulihan M, White LR, Farrer M. Clinical features of LRRK2-associated Parkinson’s disease in central Norway. Ann Neurol. 2005;57(5):762–765. - PubMed
-
- Adams JR, van Netten H, Schulzer M, Mak E, McKenzie J, Strongosky A, Sossi V, Ruth TJ, Lee CS, Farrer M, Gasser T, Uitti RJ, Calne DB, Wszolek ZK, Stoessl AJ. PET in LRRK2 mutations: comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain. 2005;128(Pt 12):2777–2785. - PubMed
-
- Berg DSK, Leitner P, Zimprich A, Lichtner P, Belcredi P, Brussel T, Schulte C, Maass S, Nagele T. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson’s disease*. Brain. 2005;128(Pt 12):3000–3011. - PubMed
-
- Bialecka M, Hui S, Klodowska-Duda G, Opala G, Tan EK, Drozdzik M. Analysis of LRRK 2 G 2019 S and I 2020 T mutations in Parkinson’s disease. Neurosci Lett. 2005;390(1):1–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

