Impaired negative regulation of homeostatically proliferating T cells
- PMID: 19023118
- PMCID: PMC2948436
- DOI: 10.1182/blood-2008-03-139964
Impaired negative regulation of homeostatically proliferating T cells
Abstract
Acute lymphopenia-induced homeostatic proliferation (HP) of T cells promotes antitumor immunity, but the mechanism is unclear. We hypothesized that this is due to a lack of inhibitory signals that allows activation of T cells with low affinity for self-antigens. Tumors resist immunity in part by expressing inhibitory molecules such as PD-1 ligand 1 (PD-L1), B7-H4, and TGF-beta. In irradiated mice undergoing HP, we found that T cells displayed a severe deficit in the activation-induced expression of inhibitory molecules PD-1 and CTLA-4, and TGF-beta1-induced expression of Foxp3. HP T cells were also less suppressed by B7-H4/Ig and, unlike control T cells, failed to produce IL-10 in response to this molecule. This deficiency in regulation was reversed as normal T-cell numbers were restored. We conclude that T cells are weakly regulated by inhibitory molecules during the acute phase of HP, which could explain their increased effectiveness in cancer immunotherapy.
Figures

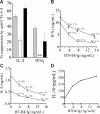
 indicates normal; □, HP 2 weeks; and ■, HP 3 weeks. Anti–CTLA-4 exerted significant inhibitory effect on normal and HP 3-week cells (P < .05). ** indicates IFNγ production was increased by 34% plus or minus 8%, rather than suppressed. (B-C) HP T cells (■) or control T cells (▲) were recovered 25 days following cell transfer and stimulated with anti-CD3/anti-CD28, in the presence or absence of B7-H4/Ig. Supernatants were assayed for IFNγ (B) and IL-2 (C). Similar results were obtained at 21 days after cell transfer (data not shown). * indicates HP T cells are significantly different from normal T cells (P < .05). (D) B7-H4/Ig costimulation enhances IL-10 production in normal (▲) but not HP T (■) cells (P < .05). The experimental protocol is same as panels B and C. The results are the mean plus or minus SEM of 3 mice.
indicates normal; □, HP 2 weeks; and ■, HP 3 weeks. Anti–CTLA-4 exerted significant inhibitory effect on normal and HP 3-week cells (P < .05). ** indicates IFNγ production was increased by 34% plus or minus 8%, rather than suppressed. (B-C) HP T cells (■) or control T cells (▲) were recovered 25 days following cell transfer and stimulated with anti-CD3/anti-CD28, in the presence or absence of B7-H4/Ig. Supernatants were assayed for IFNγ (B) and IL-2 (C). Similar results were obtained at 21 days after cell transfer (data not shown). * indicates HP T cells are significantly different from normal T cells (P < .05). (D) B7-H4/Ig costimulation enhances IL-10 production in normal (▲) but not HP T (■) cells (P < .05). The experimental protocol is same as panels B and C. The results are the mean plus or minus SEM of 3 mice.Similar articles
-
Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion.J Immunol. 2008 Oct 15;181(8):5748-59. doi: 10.4049/jimmunol.181.8.5748. J Immunol. 2008. PMID: 18832734
-
FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy.BMC Cancer. 2008 Feb 23;8:57. doi: 10.1186/1471-2407-8-57. BMC Cancer. 2008. PMID: 18294387 Free PMC article.
-
Induction of CD4 T cell changes in murine AIDS is dependent on costimulation and involves a dysregulation of homeostasis.J Immunol. 2002 Jul 15;169(2):722-31. doi: 10.4049/jimmunol.169.2.722. J Immunol. 2002. PMID: 12097374
-
Fine tuning the immune response through B7-H3 and B7-H4.Immunol Rev. 2009 May;229(1):145-51. doi: 10.1111/j.1600-065X.2009.00768.x. Immunol Rev. 2009. PMID: 19426220 Free PMC article. Review.
-
Targeting T cell costimulation in autoimmune disease.Expert Opin Ther Targets. 2002 Jun;6(3):275-89. doi: 10.1517/14728222.6.3.275. Expert Opin Ther Targets. 2002. PMID: 12223069 Review.
Cited by
-
Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma.J Immunol. 2010 Apr 1;184(7):3442-9. doi: 10.4049/jimmunol.0904114. Epub 2010 Mar 1. J Immunol. 2010. PMID: 20194714 Free PMC article.
-
Stable activity of diabetogenic cells with age in NOD mice: dynamics of reconstitution and adoptive diabetes transfer in immunocompromised mice.Immunology. 2014 Jul;142(3):465-73. doi: 10.1111/imm.12277. Immunology. 2014. PMID: 24601987 Free PMC article.
-
Phase 2 study of PD-1 blockade following autologous transplantation for patients with AML ineligible for allogeneic transplant.Blood Adv. 2023 Sep 26;7(18):5215-5224. doi: 10.1182/bloodadvances.2023010477. Blood Adv. 2023. PMID: 37379271 Free PMC article. Clinical Trial.
-
Arthritogenic T cells drive the recovery of autoantibody-producing B cell homeostasis and the adoptive transfer of arthritis in SCID mice.Int Immunol. 2012 Aug;24(8):507-17. doi: 10.1093/intimm/dxs057. Epub 2012 Apr 19. Int Immunol. 2012. PMID: 22518822 Free PMC article.
-
Quantitative monitoring of mouse lung tumors by magnetic resonance imaging.Nat Protoc. 2012 Jan 5;7(1):128-42. doi: 10.1038/nprot.2011.424. Nat Protoc. 2012. PMID: 22222788 Free PMC article.
References
-
- Baccala R, Gonzalez-Quintial R, Dummer W, et al. Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives. Springer Semin Immunopathol. 2005;27:75–85. - PubMed
-
- Neujahr DC, Chen C, Huang X, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–4639. - PubMed
-
- Borrello I, Sotomayor EM, Rattis FM, et al. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–3019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

