Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment
- PMID: 19023089
- PMCID: PMC2701301
- DOI: 10.1161/ATVBAHA.108.179218
Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment
Abstract
Objective: The lack of therapies that inhibit valvular calcification and the conflicting outcomes of clinical studies regarding the impact of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors on valve disease highlight the need for controlled investigations to characterize the interactions between HMG-CoA reductase inhibitors and valve tissue. Thus, we applied multiple in vitro disease stimuli to valvular interstitial cell (VIC) cultures and examined the impact of simvastatin treatment on VIC function.
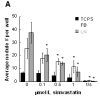
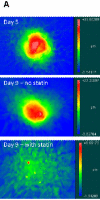
Methods and results: VICs were cultured on 3 different substrates that supported various levels of nodule formation. Transforming growth factor (TGF)-beta1 was also applied as a disease stimulus to VICs on 2-D surfaces or encapsulated in 3-D collagen gels and combined with different temporal applications of simvastatin. Simvastatin inhibited calcific nodule formation in a dose-dependent manner on all materials, although the level of statin efficacy was highly substrate-dependent. Simvastatin treatment significantly altered nodule morphology, resulting in dramatic nodule dissipation over time, also in a substrate-dependent manner. These effects were mimicked in 3-D cultures, wherein simvastatin reversed TGF-beta1-induced contraction. Decreases in nodule formation were not achieved via the HMG-CoA reductase pathway, but were correlated with decreases in ROCK activity.
Conclusions: These studies represent a significant contribution to understanding how simvastatin may impact heart valve calcification.
Figures













Similar articles
-
Paradoxical effects of statins on aortic valve myofibroblasts and osteoblasts: implications for end-stage valvular heart disease.Arterioscler Thromb Vasc Biol. 2005 Mar;25(3):592-7. doi: 10.1161/01.ATV.0000154278.01871.64. Epub 2004 Dec 23. Arterioscler Thromb Vasc Biol. 2005. PMID: 15618546
-
Transforming growth factor-β1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model.Am J Physiol Heart Circ Physiol. 2020 Nov 1;319(5):H1123-H1141. doi: 10.1152/ajpheart.00651.2019. Epub 2020 Sep 28. Am J Physiol Heart Circ Physiol. 2020. PMID: 32986963
-
Cadherin-11 regulates cell-cell tension necessary for calcific nodule formation by valvular myofibroblasts.Arterioscler Thromb Vasc Biol. 2013 Jan;33(1):114-20. doi: 10.1161/ATVBAHA.112.300278. Epub 2012 Nov 15. Arterioscler Thromb Vasc Biol. 2013. PMID: 23162011 Free PMC article.
-
Molecular mechanisms underlying the onset of degenerative aortic valve disease.J Mol Med (Berl). 2009 Jan;87(1):17-24. doi: 10.1007/s00109-008-0400-9. Epub 2008 Sep 3. J Mol Med (Berl). 2009. PMID: 18766323 Review.
-
The Pathogenesis and treatment of the valvulopathy of aortic stenosis: Beyond the SEAS.Curr Cardiol Rep. 2010 Mar;12(2):125-32. doi: 10.1007/s11886-010-0089-6. Curr Cardiol Rep. 2010. PMID: 20425167 Free PMC article. Review.
Cited by
-
Aortic valve: mechanical environment and mechanobiology.Ann Biomed Eng. 2013 Jul;41(7):1331-46. doi: 10.1007/s10439-013-0785-7. Epub 2013 Mar 21. Ann Biomed Eng. 2013. PMID: 23515935 Free PMC article. Review.
-
Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update.Circulation. 2011 Oct 18;124(16):1783-91. doi: 10.1161/CIRCULATIONAHA.110.006767. Circulation. 2011. PMID: 22007101 Free PMC article. Review. No abstract available.
-
IL-1β in atherosclerotic vascular calcification: From bench to bedside.Int J Biol Sci. 2021 Oct 22;17(15):4353-4364. doi: 10.7150/ijbs.66537. eCollection 2021. Int J Biol Sci. 2021. PMID: 34803503 Free PMC article. Review.
-
Bicuspid aortic valve disease: the role of oxidative stress in Lrp5 bone formation.Cardiovasc Pathol. 2011 May-Jun;20(3):168-76. doi: 10.1016/j.carpath.2010.11.007. Epub 2011 Jan 22. Cardiovasc Pathol. 2011. PMID: 21257323 Free PMC article. Review.
-
Characterization of Laminins in Healthy Human Aortic Valves and a Modified Decellularized Rat Scaffold.Biores Open Access. 2020 Dec 7;9(1):269-278. doi: 10.1089/biores.2020.0018. eCollection 2020. Biores Open Access. 2020. PMID: 33376633 Free PMC article.
References
-
- Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–1080. - PubMed
-
- Mohler ER, 3rd, Wang H, Medenilla E, Scott C. Effect of statin treatment on aortic valve and coronary artery calcification. J Heart Valve Dis. 2007;16:378–386. - PubMed
-
- Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. - PubMed
-
- Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–466. - PubMed
-
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

