Annexin A2 at the interface between F-actin and membranes enriched in phosphatidylinositol 4,5,-bisphosphate
- PMID: 19022301
- PMCID: PMC7611824
- DOI: 10.1016/j.bbamcr.2008.10.007
Annexin A2 at the interface between F-actin and membranes enriched in phosphatidylinositol 4,5,-bisphosphate
Abstract
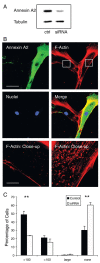
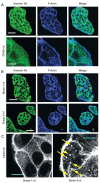
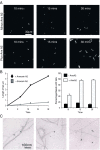
Vesicle rocketing has been used as a model system for understanding the dynamics of the membrane-associated F-actin cytoskeleton, but in many experimental systems is induced by persistent, non-physiological stimuli. Localised changes in the concentration of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in membranes stimulate the recruitment of actin-remodelling proteins to their sites of action, regulate their activity and favour vesicle rocketing. The calcium and anionic phospholipid-binding protein annexin A2 is necessary for macropinocytic rocketing and has been shown to bind both PI(4,5)P2 and the barbed-ends of F-actin filaments. Here we show that annexin A2 localises to the comet tails which form constitutively in fibroblasts from patients with Lowe Syndrome. These fibroblasts are deficient in OCRL1, a phosphatidylinositol polyphosphate 5-phosphatase with specificity for PI(4,5)P2. We show that upon depletion of annexin A2 from these cells vesicle rocketing is reduced, and that this is also dependent upon PI(4,5)P2 formation. Annexin A2 co-localised with comet-tails induced by pervanadate and hyperosmotic shock in a basophilic cell line, and in an epithelial cell line upon activation of PKC. In vitro annexin A2 promoted comet formation in a bead-rocketing assay and was sufficient to link F-actin filaments to PI(4,5)P2 containing vesicles. These observations are consistent with a role for annexin A2 as an actin nucleator on PI(4,5)P2-enriched membranes.
Figures

Similar articles
-
Annexin 2 binding to phosphatidylinositol 4,5-bisphosphate on endocytic vesicles is regulated by the stress response pathway.J Biol Chem. 2004 Apr 2;279(14):14157-64. doi: 10.1074/jbc.M313025200. Epub 2004 Jan 20. J Biol Chem. 2004. PMID: 14734570 Free PMC article.
-
Lipid segregation and membrane budding induced by the peripheral membrane binding protein annexin A2.J Biol Chem. 2013 Aug 23;288(34):24764-76. doi: 10.1074/jbc.M113.474023. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861394 Free PMC article.
-
Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes.J Cell Sci. 2004 Jul 15;117(Pt 16):3473-80. doi: 10.1242/jcs.01208. Epub 2004 Jun 29. J Cell Sci. 2004. PMID: 15226372
-
Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms.Biochem Biophys Res Commun. 2018 Nov 25;506(2):307-314. doi: 10.1016/j.bbrc.2018.07.155. Epub 2018 Aug 13. Biochem Biophys Res Commun. 2018. PMID: 30139519 Free PMC article. Review.
-
The cellular and physiological functions of the Lowe syndrome protein OCRL1.Traffic. 2014 May;15(5):471-87. doi: 10.1111/tra.12160. Epub 2014 Mar 7. Traffic. 2014. PMID: 24499450 Free PMC article. Review.
Cited by
-
Optogenetic Modulation of Intraocular Pressure in a Glucocorticoid-Induced Ocular Hypertension Mouse Model.Transl Vis Sci Technol. 2021 May 3;10(6):10. doi: 10.1167/tvst.10.6.10. Transl Vis Sci Technol. 2021. PMID: 34111256 Free PMC article.
-
Annexin 2 has a dual role as regulator and effector of v-Src in cell transformation.J Biol Chem. 2009 Apr 10;284(15):10202-10. doi: 10.1074/jbc.M807043200. Epub 2009 Feb 4. J Biol Chem. 2009. PMID: 19193640 Free PMC article.
-
Recombinant Annexin A2 Administration Improves Neurological Outcomes After Traumatic Brain Injury in Mice.Front Pharmacol. 2021 Jul 12;12:708469. doi: 10.3389/fphar.2021.708469. eCollection 2021. Front Pharmacol. 2021. PMID: 34400908 Free PMC article.
-
Annexin A2 promotes phagophore assembly by enhancing Atg16L⁺ vesicle biogenesis and homotypic fusion.Nat Commun. 2015 Jan 19;6:5856. doi: 10.1038/ncomms6856. Nat Commun. 2015. PMID: 25597631 Free PMC article.
-
Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2.J Biol Chem. 2011 Sep 2;286(35):30911-30925. doi: 10.1074/jbc.M111.271155. Epub 2011 Jul 7. J Biol Chem. 2011. PMID: 21737841 Free PMC article.
References
-
- Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. - PubMed
-
- Girao H, Geli MI, Idrissi FZ. Actin in the endocytic pathway: from yeast to mammals. FEBS Lett. 2008;582:2112–2119. - PubMed
-
- De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. - PubMed
-
- Cameron LA, Giardini PA, Soo FS, Theriot JA. Secrets of actin-based motility revealed by a bacterial pathogen. Nat Rev Mol Cell Biol. 2000;1:110–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

