Differential effector mechanisms induced by vaccination with MUC1 DNA in the rejection of colon carcinoma growth at orthotopic sites and metastases
- PMID: 19018774
- PMCID: PMC11158969
- DOI: 10.1111/j.1349-7006.2008.00967.x
Differential effector mechanisms induced by vaccination with MUC1 DNA in the rejection of colon carcinoma growth at orthotopic sites and metastases
Abstract
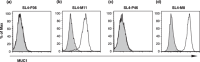
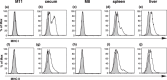
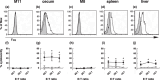
The effects of MUC1 DNA vaccination on the orthotopic growth and liver metastasis of colon carcinoma cells were investigated in mice. Vaccination with MUC1 DNA resulted in immune responses that were effective in suppressing mouse colon carcinoma cells transfected with MUC1 cDNA. CD4+ T cells but not CD8+ T cells mediated this antitumor response as shown by the in vivo depletion of lymphocyte subpopulations with the use of anti-CD4 or anti-CD8 antibody. The effects of neutralizing antibodies in vivo revealed that the predominant effector molecule in preventing orthotopic tumor growth was FasL, whereas the effector molecule effective in preventing liver metastasis was tumor necrosis factor-alpha. Colon carcinoma cells isolated from tumors growing in the ceca, spleens, and livers were shown to be equally sensitive to FasL and tumor necrosis factor-alpha. The results strongly suggest that elimination of tumor cells initiated by DNA vaccination in the present protocol is mediated by antigen-specific CD4+ T cells and the effector mechanisms in the cecum and in the liver are distinct due to a unique organ microenvironment.
Figures





Similar articles
-
Vaccination of mice with MUC1 cDNA suppresses the development of lung metastases.Clin Exp Metastasis. 2002;19(8):689-96. doi: 10.1023/a:1021332932531. Clin Exp Metastasis. 2002. PMID: 12553374
-
Organ microenvironment plays significant roles through Fas ligand in vaccine-induced CD4(+) T cell dependent suppression of tumor growth at the orthotopic site.Cancer Sci. 2010 Sep;101(9):1965-9. doi: 10.1111/j.1349-7006.2010.01634.x. Cancer Sci. 2010. PMID: 20637014 Free PMC article.
-
Molecular requirements for CD8-mediated rejection of a MUC1-expressing pancreatic carcinoma: implications for tumor vaccines.Cancer Immunol Immunother. 2002 Aug;51(6):327-40. doi: 10.1007/s00262-002-0277-3. Epub 2002 May 4. Cancer Immunol Immunother. 2002. PMID: 12111121 Free PMC article.
-
Intradermal vaccination of MUC1 transgenic mice with MUC1/IL-18 plasmid DNA suppresses experimental pulmonary metastases.Vaccine. 2007 Apr 30;25(17):3338-46. doi: 10.1016/j.vaccine.2007.01.007. Epub 2007 Jan 22. Vaccine. 2007. PMID: 17292519
-
Novel vaccination protocol consisting of injecting MUC1 DNA and nonprimed dendritic cells at the same region greatly enhanced MUC1-specific antitumor immunity in a murine model.Cancer Gene Ther. 2002 Apr;9(4):330-7. doi: 10.1038/sj.cgt.7700444. Cancer Gene Ther. 2002. PMID: 11960283
Cited by
-
MUC1 is responsible for the pro-metastatic potential of calycosin in pancreatic ductal adenocarcinoma.Am J Cancer Res. 2022 Jul 15;12(7):3242-3258. eCollection 2022. Am J Cancer Res. 2022. PMID: 35968328 Free PMC article.
-
Induction of antigen-specific CTL and antibody responses in mice by a novel recombinant tandem repeat DNA vaccine targeting at mucin 1 of pancreatic cancer.J Cancer Res Clin Oncol. 2010 Dec;136(12):1861-8. doi: 10.1007/s00432-010-0845-4. Epub 2010 Mar 13. J Cancer Res Clin Oncol. 2010. PMID: 20229033
-
Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells.Cancers (Basel). 2023 Mar 22;15(6):1920. doi: 10.3390/cancers15061920. Cancers (Basel). 2023. PMID: 36980805 Free PMC article.
-
Mucin1 as a potential molecule for cancer immunotherapy and targeted therapy.J Cancer. 2024 Jan 1;15(1):54-67. doi: 10.7150/jca.88261. eCollection 2024. J Cancer. 2024. PMID: 38164273 Free PMC article. Review.
-
CD4+ and CD8+ T cells can act separately in tumour rejection after immunization with murine pneumotropic virus chimeric Her2/neu virus-like particles.PLoS One. 2010 Jul 19;5(7):e11580. doi: 10.1371/journal.pone.0011580. PLoS One. 2010. PMID: 20657846 Free PMC article.
References
-
- Fidler IJ, Kim SJ, Langley RR. The role of the organ microenvironment in the biology and therapy of cancer metastasis. J Cell Biochem 2007; 101: 927–36. - PubMed
-
- Kubota T. Metastatic models of human cancer xenografted in the nude mouse: the importance of orthotopic transplantation. J Cell Biochem 1994; 56: 4–8. - PubMed
-
- Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 1994; 106: 353–61. - PubMed
-
- Denda‐Nagai K, Irimura T. MUC1 in carcinoma–host interactions. Glycoconj J 2000; 17: 649–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

