TNF receptor-associated factor 5 is required for optimal T cell expansion and survival in response to infection
- PMID: 19017969
- PMCID: PMC2636746
- DOI: 10.4049/jimmunol.181.11.7800
TNF receptor-associated factor 5 is required for optimal T cell expansion and survival in response to infection
Abstract
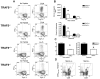
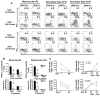
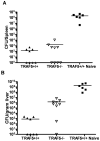
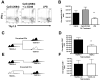
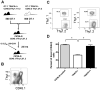
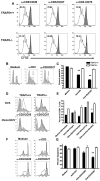
Receptors belonging to the TNF-receptor (TNF-R) superfamily include important costimulatory molecules, many of which specifically affect T cell activation. TNF receptor-associated factors (TRAFs) are recruited to many TNF-R superfamily members and are important modulators of the proximal signaling events that occur at the time of receptor engagement and activation. TRAF5 has been shown to be a positive regulator of a number of these receptors that are involved in T cell costimulation. However, the potential importance of TRAF5 in cellular immune responses to infection or in T cell expansion and memory have not been studied. We report in this study that TRAF5 was required for optimal CD8(+) T cell responses following infection with Listeria monocytogenes expressing OVA (LM-OVA). TRAF5 was necessary for optimal T cell expansion following primary infection with LM-OVA, and its absence resulted in fewer memory CD8(+) T cells following LM-OVA infection, together with higher bacterial loads in the liver. The effect of TRAF5 on CD8(+) T cell expansion was T cell intrinsic and not due to effects of TRAF5 deficiency on APCs. Although their proliferative ability remained intact, CD8(+) T cells from TRAF5(-/-) mice were more sensitive to apoptosis and were unresponsive to the prosurvival effects of the TNF-R superfamily costimulator CD27. Collectively, these studies identify TRAF5 as an important positive signaling element that enhances T cell expansion and pathogen containment by providing a survival advantage to responding Ag-specific CD8(+) T cells during infection.
Figures
Similar articles
-
Prolonged antigen presentation, APC-, and CD8+ T cell turnover during mycobacterial infection: comparison with Listeria monocytogenes.J Immunol. 2004 Mar 15;172(6):3491-500. doi: 10.4049/jimmunol.172.6.3491. J Immunol. 2004. PMID: 15004149
-
Imatinib mesylate inhibits antigen-specific memory CD8 T cell responses in vivo.J Immunol. 2007 Feb 15;178(4):2028-37. doi: 10.4049/jimmunol.178.4.2028. J Immunol. 2007. PMID: 17277106
-
Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection.J Immunol. 2003 Feb 15;170(4):2053-63. doi: 10.4049/jimmunol.170.4.2053. J Immunol. 2003. PMID: 12574376
-
Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions.Immunol Rev. 2011 Nov;244(1):55-74. doi: 10.1111/j.1600-065X.2011.01055.x. Immunol Rev. 2011. PMID: 22017431 Free PMC article. Review.
-
[Immune Regulation by TNF Receptor-associated Factor 5].Yakugaku Zasshi. 2024;144(5):489-496. doi: 10.1248/yakushi.23-00154-3. Yakugaku Zasshi. 2024. PMID: 38692922 Review. Japanese.
Cited by
-
TRAF2 exerts opposing effects on basal and TNFα-induced activation of the classic IKK complex in hematopoietic cells in mice.J Cell Sci. 2016 Apr 1;129(7):1455-67. doi: 10.1242/jcs.180554. Epub 2016 Feb 12. J Cell Sci. 2016. PMID: 26872784 Free PMC article.
-
TRAF5-mediated Lys-63-linked Polyubiquitination Plays an Essential Role in Positive Regulation of RORγt in Promoting IL-17A Expression.J Biol Chem. 2015 Nov 27;290(48):29086-94. doi: 10.1074/jbc.M115.664573. Epub 2015 Oct 9. J Biol Chem. 2015. PMID: 26453305 Free PMC article.
-
Screening of JAK-STAT modulators from the antiviral plants of Indian traditional system of medicine with the potential to inhibit 2019 novel coronavirus using network pharmacology.3 Biotech. 2021 Mar;11(3):119. doi: 10.1007/s13205-021-02664-4. Epub 2021 Feb 8. 3 Biotech. 2021. PMID: 33585152 Free PMC article.
-
Spatiotemporal patterns and essential role of TNF receptor-associated factor 5 expression after rat spinal cord Injury.J Mol Histol. 2012 Oct;43(5):527-33. doi: 10.1007/s10735-012-9411-5. Epub 2012 Apr 8. J Mol Histol. 2012. PMID: 22484641
-
Notch controls the magnitude of T helper cell responses by promoting cellular longevity.Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):9041-6. doi: 10.1073/pnas.1206044109. Epub 2012 May 21. Proc Natl Acad Sci U S A. 2012. PMID: 22615412 Free PMC article.
References
-
- Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. - PubMed
-
- Flynn K, Mullbacher A. The generation of memory antigen-specific cytotoxic T cell responses by CD28/CD80 interactions in the absence of antigen. Eur J Immunol. 1997;27:456–462. - PubMed
-
- Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. - PubMed
-
- Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 Promotes Bcl-xL and Bcl-2 Expression and Is Essential for Long-Term Survival of CD4 T Cells. Immunity. 2001;15:445–455. - PubMed
-
- Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine & Growth Factor Reviews The TNF Superfamily. 2003;14:265–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

