Discovery and development of heat shock protein 90 inhibitors
- PMID: 19017562
- PMCID: PMC2760286
- DOI: 10.1016/j.bmc.2008.10.087
Discovery and development of heat shock protein 90 inhibitors
Abstract
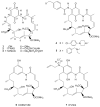
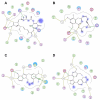
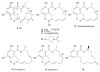
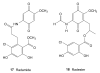
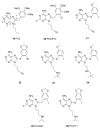
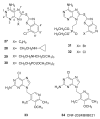
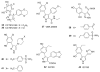
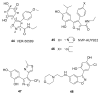
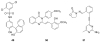
Heat shock protein 90 (Hsp90) is an important target in cancer because of its role in maintaining transformation and has recently become the focus of several drug discovery and development efforts. While compounds with different modes of action are known, the focus of this review is on those classes of compounds which inhibit Hsp90 by binding to the N-terminal ATP pocket. These include natural product inhibitors such as geldanamycin and radicicol and synthetic inhibitors comprised of purines, pyrazoles, isoxazoles and other scaffolds. The synthetic inhibitors have been discovered either by structure-based design, high throughput screening and more recently using fragment-based design and virtual screening techniques. This review will discuss the discovery of these different classes, as well as their development as potential clinical agents.
Figures
Similar articles
-
Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 N-terminal ATP binding site.Curr Top Med Chem. 2006;6(11):1173-82. doi: 10.2174/156802606777812031. Curr Top Med Chem. 2006. PMID: 16842154 Review.
-
C-terminal modulators of heat shock protein of 90 kDa (HSP90): State of development and modes of action.Bioorg Med Chem. 2019 Nov 1;27(21):115080. doi: 10.1016/j.bmc.2019.115080. Epub 2019 Aug 26. Bioorg Med Chem. 2019. PMID: 31519378 Review.
-
Discovery of benzisoxazoles as potent inhibitors of chaperone heat shock protein 90.J Med Chem. 2008 Feb 14;51(3):373-5. doi: 10.1021/jm701385c. Epub 2008 Jan 16. J Med Chem. 2008. PMID: 18197612
-
Fragment screening using capillary electrophoresis (CEfrag) for hit identification of heat shock protein 90 ATPase inhibitors.J Biomol Screen. 2012 Aug;17(7):868-76. doi: 10.1177/1087057112445785. Epub 2012 May 9. J Biomol Screen. 2012. PMID: 22573733
-
Natural Product Inspired N-Terminal Hsp90 Inhibitors: From Bench to Bedside?Med Res Rev. 2016 Jan;36(1):92-118. doi: 10.1002/med.21351. Epub 2015 May 25. Med Res Rev. 2016. PMID: 26010985 Free PMC article. Review.
Cited by
-
Understanding the Physical and Molecular Basis of Stability of Arabidopsis DNA Pol λ under UV-B and High NaCl Stress.PLoS One. 2015 Jul 31;10(7):e0133843. doi: 10.1371/journal.pone.0133843. eCollection 2015. PLoS One. 2015. PMID: 26230318 Free PMC article.
-
Celastrol inhibits Hsp90 chaperoning of steroid receptors by inducing fibrillization of the Co-chaperone p23.J Biol Chem. 2010 Feb 5;285(6):4224-4231. doi: 10.1074/jbc.M109.081018. Epub 2009 Dec 8. J Biol Chem. 2010. PMID: 19996313 Free PMC article.
-
The Chemical Biology of Molecular Chaperones--Implications for Modulation of Proteostasis.J Mol Biol. 2015 Sep 11;427(18):2931-47. doi: 10.1016/j.jmb.2015.05.010. Epub 2015 May 21. J Mol Biol. 2015. PMID: 26003923 Free PMC article. Review.
-
Structural ensemble-based docking simulation and biophysical studies discovered new inhibitors of Hsp90 N-terminal domain.Sci Rep. 2018 Jan 10;8(1):368. doi: 10.1038/s41598-017-18332-8. Sci Rep. 2018. PMID: 29321504 Free PMC article.
-
Molecular chaperones as rational drug targets for Parkinson's disease therapeutics.CNS Neurol Disord Drug Targets. 2010 Dec;9(6):741-53. doi: 10.2174/187152710793237386. CNS Neurol Disord Drug Targets. 2010. PMID: 20942788 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

