An essential role for the Glut1 PDZ-binding motif in growth factor regulation of Glut1 degradation and trafficking
- PMID: 19016655
- PMCID: PMC2637307
- DOI: 10.1042/BJ20081422
An essential role for the Glut1 PDZ-binding motif in growth factor regulation of Glut1 degradation and trafficking
Abstract
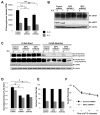
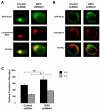
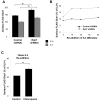
Cell surface localization of the Glut (glucose transporter), Glut1, is a cytokine-controlled process essential to support the metabolism and survival of haemopoietic cells. Molecular mechanisms that regulate Glut1 trafficking, however, are not certain. In the present study, we show that a C-terminal PDZ-binding motif in Glut1 is critical to promote maximal cytokine-stimulated Glut1 cell surface localization and prevent Glut1 lysosomal degradation in the absence of growth factor. Disruption of this PDZ-binding sequence through deletion or point mutation sharply decreased surface Glut1 levels and led to rapid targeting of internalized Glut1 to lysosomes for proteolysis, particularly in growth factor-deprived cells. The PDZ-domain protein, GIPC (G(alpha)-interacting protein-interacting protein, C-terminus), bound to Glut1 in part via the Glut1 C-terminal PDZ-binding motif, and we found that GIPC deficiency decreased Glut1 surface levels and glucose uptake. Unlike the Glut1 degradation observed on mutation of the Glut1 PDZ-binding domain, however, GIPC deficiency resulted in accumulation of intracellular Glut1 in a pool distinct from the recycling pathway of the TfR (transferrin receptor). Blockade of Glut1 lysosomal targeting after growth factor withdrawal also led to intracellular accumulation of Glut1, a portion of which could be rapidly restored to the cell surface after growth factor stimulation. These results indicate that the C-terminal PDZ-binding motif of Glut1 plays a key role in growth factor regulation of glucose uptake by both allowing GIPC to promote Glut1 trafficking to the cell surface and protecting intracellular Glut1 from lysosomal degradation after growth factor withdrawal, thus allowing the potential for a rapid return of intracellular Glut1 to the cell surface on restimulation.
Figures

Similar articles
-
Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking.Mol Biol Cell. 2007 Apr;18(4):1437-46. doi: 10.1091/mbc.e06-07-0593. Epub 2007 Feb 14. Mol Biol Cell. 2007. PMID: 17301289 Free PMC article.
-
GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP.Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12340-5. doi: 10.1073/pnas.95.21.12340. Proc Natl Acad Sci U S A. 1998. PMID: 9770488 Free PMC article.
-
Effect of plasma membrane cholesterol depletion on glucose transport regulation in leukemia cells.PLoS One. 2012;7(7):e41246. doi: 10.1371/journal.pone.0041246. Epub 2012 Jul 30. PLoS One. 2012. PMID: 22859971 Free PMC article.
-
PDZ Protein Regulation of G Protein-Coupled Receptor Trafficking and Signaling Pathways.Mol Pharmacol. 2015 Oct;88(4):624-39. doi: 10.1124/mol.115.098509. Epub 2015 Mar 25. Mol Pharmacol. 2015. PMID: 25808930 Review.
-
Functional proteomics, human genetics and cancer biology of GIPC family members.Exp Mol Med. 2013 Jun 7;45(6):e26. doi: 10.1038/emm.2013.49. Exp Mol Med. 2013. PMID: 23743496 Free PMC article. Review.
Cited by
-
The adaptor protein GIPC1 stabilizes the scavenger receptor SR-B1 and increases its cholesterol uptake.J Biol Chem. 2021 Jan-Jun;296:100616. doi: 10.1016/j.jbc.2021.100616. Epub 2021 Mar 31. J Biol Chem. 2021. PMID: 33811857 Free PMC article.
-
Expression and distribution of trophoblast glycoprotein in the mouse retina.J Comp Neurol. 2020 Jul;528(10):1660-1671. doi: 10.1002/cne.24850. Epub 2020 Jan 6. J Comp Neurol. 2020. PMID: 31891182 Free PMC article.
-
The metabolic life and times of a T-cell.Immunol Rev. 2010 Jul;236:190-202. doi: 10.1111/j.1600-065X.2010.00911.x. Immunol Rev. 2010. PMID: 20636818 Free PMC article. Review.
-
Integrin alpha5beta1 function is regulated by XGIPC/kermit2 mediated endocytosis during Xenopus laevis gastrulation.PLoS One. 2010 May 17;5(5):e10665. doi: 10.1371/journal.pone.0010665. PLoS One. 2010. PMID: 20498857 Free PMC article.
-
GAIP interacting protein C-terminus regulates autophagy and exosome biogenesis of pancreatic cancer through metabolic pathways.PLoS One. 2014 Dec 3;9(12):e114409. doi: 10.1371/journal.pone.0114409. eCollection 2014. PLoS One. 2014. PMID: 25469510 Free PMC article.
References
-
- Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. - PubMed
-
- Bentley J, Itchayanan D, Barnes K, McIntosh E, Tang X, Downes CP, Holman GD, Whetton AD, Owen-Lynch PJ, Baldwin SA. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J Biol Chem. 2003;278:39337–39348. - PubMed
-
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

