In vitro and in vivo evidence for stimulation of bone resorption by an EP4 receptor agonist and basic fibroblast growth factor: Implications for their efficacy as bone anabolic agents
- PMID: 19013265
- PMCID: PMC2663525
- DOI: 10.1016/j.bone.2008.10.041
In vitro and in vivo evidence for stimulation of bone resorption by an EP4 receptor agonist and basic fibroblast growth factor: Implications for their efficacy as bone anabolic agents
Abstract
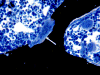
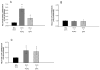
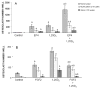
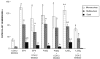
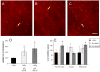
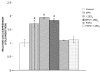
Prostaglandin E2 receptor subtype 4 agonists (EP4A) and basic fibroblast growth factor (FGF2) stimulate bone formation, but their effects on bone resorption are controversial. To provide additional insight into the skeletal effects of EP4A and FGF2, their regulation of expression of genes associated with bone formation and resorption in aged ovariectomized (OVX) rats and in cultured mouse bone marrow cells was determined. RNA was isolated from lumbar vertebrae of OVX rats (16 months of age) treated daily for 3 weeks with FGF2 or EP4A and processed for quantitative real time-PCR analyses. mRNA expression for the receptor activator of NF-kappaB ligand (RANKL) and cathepsin K (CTSK), but not osteoprotegerin (OPG), were upregulated by both FGF2 and EP4A. Addition of FGF2 and EP4A to the medium of cultured mouse bone marrow cells increased the formation of tartrate resistant acid phosphatase (TRAP) positive cells, upregulated the expression of RANKL and CTSK, and downregulated expression for OPG. EP4A also increased the formation of actin rings, an indicator of osteoclast activation, in a dose dependent manner in osteoclasts cultured on bone slices and triggered the formation of pits as revealed by a pitting assay. Gene expression for osterix (OSX) and IGF-2, genes associated with bone formation, was significantly greater in FGF2-treated OVX rats compared with EP4A-treated OVX rats. These findings at the molecular level are consistent with previous tissue-level histomorphometric findings, and at the doses tested, support the contention that FGF2 has a stronger bone anabolic effect than EP4A. The results of these in vivo and in vitro analyses clarify the effects of FGF2 and EP4A on bone formation and resorption, and provide insight into differences in the efficacy of two potential bone anabolic agents for restoration of lost bone mass in the osteopenic, estrogen-deplete skeleton.
Figures
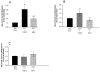
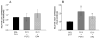

Similar articles
-
Effects of basic fibroblast growth factor and a prostaglandin E2 receptor subtype 4 agonist on osteoblastogenesis and adipogenesis in aged ovariectomized rats.J Bone Miner Res. 2007 Jun;22(6):877-88. doi: 10.1359/jbmr.070313. J Bone Miner Res. 2007. PMID: 17352655
-
Effects of selective prostaglandin EP4 receptor antagonist on osteoclast formation and bone resorption in vitro.Bone. 2002 Jan;30(1):159-63. doi: 10.1016/s8756-3282(01)00688-3. Bone. 2002. PMID: 11792579
-
Osteopontin deficiency enhances anabolic action of EP4 agonist at a sub-optimal dose in bone.J Endocrinol. 2007 Apr;193(1):171-82. doi: 10.1677/joe.1.06917. J Endocrinol. 2007. PMID: 17400814
-
Oral administration of prostaglandin E(2)-specific receptor 4 antagonist inhibits lipopolysaccharide-induced osteoclastogenesis in rat periodontal tissue.J Periodontol. 2012 Apr;83(4):506-13. doi: 10.1902/jop.2011.110301. Epub 2011 Sep 12. J Periodontol. 2012. PMID: 21910594
-
A comparative study of the bone-restorative efficacy of anabolic agents in aged ovariectomized rats.Osteoporos Int. 2007 Mar;18(3):351-62. doi: 10.1007/s00198-006-0240-9. Epub 2006 Nov 22. Osteoporos Int. 2007. PMID: 17120182
Cited by
-
The Crossroads between Infection and Bone Loss.Microorganisms. 2020 Nov 10;8(11):1765. doi: 10.3390/microorganisms8111765. Microorganisms. 2020. PMID: 33182721 Free PMC article. Review.
-
Prostaglandin EP4 Selective Agonist AKDS001 Enhances New Bone Formation by Minimodeling in a Rat Heterotopic Xenograft Model of Human Bone.Front Bioeng Biotechnol. 2022 Mar 17;10:845716. doi: 10.3389/fbioe.2022.845716. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35372320 Free PMC article.
-
In vivo identification of Bmp2-correlation networks during fracture healing by means of a limb-specific conditional inactivation of Bmp2.Bone. 2018 Nov;116:103-110. doi: 10.1016/j.bone.2018.07.016. Epub 2018 Jul 23. Bone. 2018. PMID: 30048819 Free PMC article.
-
Effectiveness of combined salmon calcitonin and aspirin therapy for osteoporosis in ovariectomized rats.Mol Med Rep. 2015 Aug;12(2):1717-26. doi: 10.3892/mmr.2015.3637. Epub 2015 Apr 16. Mol Med Rep. 2015. PMID: 25891179 Free PMC article.
-
Effects of a sulfated exopolysaccharide produced by Altermonas infernus on bone biology.Glycobiology. 2011 Jun;21(6):781-95. doi: 10.1093/glycob/cwr002. Epub 2011 Mar 8. Glycobiology. 2011. PMID: 21385793 Free PMC article.
References
-
- Consensus Development Conference V. Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1994;90:646–650. - PubMed
-
- Foundation NO. America’s Bone Health: The state of osteoporosis and low bone mass in our nation. National Osteoporosis Foundation; 2000.
-
- Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocrine Rev. 2005;26:688–703. - PubMed
-
- Qi H, Li M, Wronski TJ. Comparison of the bone anabolic effects effects of parathyroid hormone at skeletal sites with moderate and severe osteopenia in aged ovariectomized rats. J Bone Miner Res. 1995;10:948–955. - PubMed
-
- Zerwekh JE, Hagler HK, Sakhaee K, Gottschalk F, Peterson RD, Pak CYC. Effects of slow release sodium fluoride on cancellous bone histology and connectivity in osteoporosis. Bone. 1994;15:691–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

