Amifostine reduces lung vascular permeability via suppression of inflammatory signalling
- PMID: 19010997
- PMCID: PMC3661204
- DOI: 10.1183/09031936.00014808
Amifostine reduces lung vascular permeability via suppression of inflammatory signalling
Abstract
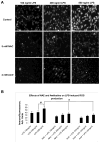
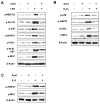
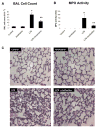
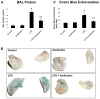
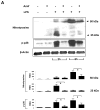
Despite an encouraging outcome of antioxidant therapy in animal models of acute lung injury, effective antioxidant agents for clinical application remain to be developed. The present study investigated the effect of pre-treatment with amifostine, a thiol antioxidant compound, on lung endothelial barrier dysfunction induced by Gram-negative bacteria wall-lipopolysaccharide (LPS). Endothelial permeability was monitored by changes in transendothelial electrical resistance. Cytoskeletal remodelling and reactive oxygen species (ROS) production was examined by immunofluorescence. Cell signalling was assessed by Western blot. Measurements of Evans blue extravasation, cell count and protein content in bronchoalveolar lavage fluid were used as in vivo parameters of lung vascular permeability. Hydrogen peroxide, LPS and interleukin-6 caused cytoskeletal reorganisation and increased permeability in the pulmonary endothelial cells, reflecting endothelial barrier dysfunction. These disruptive effects were inhibited by pre-treatment with amifostine and linked to the amifostine-mediated abrogation of ROS production and redox-sensitive signalling cascades, including p38, extracellular signal regulated kinase 1/2, mitogen-activated protein kinases and the nuclear factor-kappaB pathway. In vivo, concurrent amifostine administration inhibited LPS-induced oxidative stress and p38 mitogen-activated protein kinase activation, which was associated with reduced vascular leak and neutrophil recruitment to the lungs. The present study demonstrates, for the first time, protective effects of amifostine against lipopolysaccharide-induced lung vascular leak in vitro and in animal models of lipopolysaccharide-induced acute lung injury.
Figures



Similar articles
-
Iloprost improves endothelial barrier function in lipopolysaccharide-induced lung injury.Eur Respir J. 2013 Jan;41(1):165-76. doi: 10.1183/09031936.00148311. Epub 2012 Jul 12. Eur Respir J. 2013. PMID: 22790920 Free PMC article.
-
Induction of cellular antioxidant defense by amifostine improves ventilator-induced lung injury.Crit Care Med. 2011 Dec;39(12):2711-21. doi: 10.1097/CCM.0b013e3182284a5f. Crit Care Med. 2011. PMID: 21765345 Free PMC article.
-
Atrial natriuretic peptide attenuates LPS-induced lung vascular leak: role of PAK1.Am J Physiol Lung Cell Mol Physiol. 2010 Nov;299(5):L652-63. doi: 10.1152/ajplung.00202.2009. Epub 2010 Aug 20. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20729389 Free PMC article.
-
Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus.Am J Physiol Lung Cell Mol Physiol. 2015 Mar 15;308(6):L550-62. doi: 10.1152/ajplung.00248.2014. Epub 2015 Jan 9. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25575515 Free PMC article.
-
Opposite effects of ANP receptors in attenuation of LPS-induced endothelial permeability and lung injury.Microvasc Res. 2012 Mar;83(2):194-9. doi: 10.1016/j.mvr.2011.09.012. Epub 2011 Oct 6. Microvasc Res. 2012. PMID: 22001395 Free PMC article.
Cited by
-
Iloprost improves endothelial barrier function in lipopolysaccharide-induced lung injury.Eur Respir J. 2013 Jan;41(1):165-76. doi: 10.1183/09031936.00148311. Epub 2012 Jul 12. Eur Respir J. 2013. PMID: 22790920 Free PMC article.
-
Amifostine ameliorates bleomycin-induced murine pulmonary fibrosis via NAD+/SIRT1/AMPK pathway-mediated effects on mitochondrial function and cellular metabolism.Eur J Med Res. 2024 Jan 20;29(1):68. doi: 10.1186/s40001-023-01623-4. Eur J Med Res. 2024. PMID: 38245795 Free PMC article.
-
Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability.Am J Physiol Lung Cell Mol Physiol. 2010 Jun;298(6):L837-48. doi: 10.1152/ajplung.00263.2009. Epub 2010 Mar 26. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20348280 Free PMC article.
-
Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization.Am J Respir Cell Mol Biol. 2012 Nov;47(5):688-97. doi: 10.1165/rcmb.2012-0161OC. Epub 2012 Jul 27. Am J Respir Cell Mol Biol. 2012. PMID: 22842495 Free PMC article.
-
Anti-Inflammatory Effects of OxPAPC Involve Endothelial Cell-Mediated Generation of LXA4.Circ Res. 2017 Jul 21;121(3):244-257. doi: 10.1161/CIRCRESAHA.116.310308. Epub 2017 May 18. Circ Res. 2017. PMID: 28522438 Free PMC article.
References
-
- Baldwin SR, Simon RH, Grum CM, Ketai LH, Boxer LA, Devall LJ. Oxidant activity in expired breath of patients with adult respiratory distress syndrome. Lancet. 1986;1(8471):11–4. - PubMed
-
- Antczak A, Nowak D, Bialasiewicz P, Kasielski M. Hydrogen peroxide in expired air condensate correlates positively with early steps of peripheral neutrophil activation in asthmatic patients. Arch Immunol Ther Exp (Warsz) 1999;47(2):119–26. - PubMed
-
- Terada LS. Oxidative stress and endothelial activation. Crit Care Med. 2002;30(5 Suppl):S186–91. - PubMed
-
- Fialkow L, Chan CK, Grinstein S, Downey GP. Regulation of tyrosine phosphorylation in neutrophils by the NADPH oxidase. Role of reactive oxygen intermediates. J Biol Chem. 1993;268(23):17131–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
