Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells
- PMID: 19009526
- PMCID: PMC2734463
- DOI: 10.1002/eji.200838432
Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells
Abstract
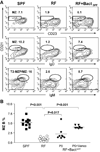
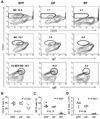
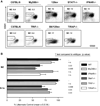
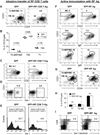
Since enteric microbial composition is a distinctive and stable individual trait, microbial heterogeneity may confer lifelong, non-genetic differences between individuals. Here we report that C57BL/6 mice bearing restricted flora microbiota, a distinct but diverse resident enteric microbial community, are numerically and functionally deficient in marginal zone (MZ) B cells. Surprisingly, MZ B-cell levels are minimally affected by germ-free conditions or null mutations of various TLR signaling molecules. In contrast, MZ B-cell depletion is exquisitely dependent on cytolytic CD8(+) T cells, and includes targeting of a cross-reactive microbial/endogenous MHC class 1B antigen. Thus, members of certain enteric microbial communities link with CD8(+) T cells as a previously unappreciated mechanism that shapes innate immunity dependent on innate-like B cells.
Figures



Similar articles
-
Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells.J Immunol. 2010 Feb 1;184(3):1218-26. doi: 10.4049/jimmunol.0902620. Epub 2010 Jan 4. J Immunol. 2010. PMID: 20048124 Free PMC article.
-
Analysis of marginal zone B cell development in the mouse with limited B cell diversity: role of the antigen receptor signals in the recruitment of B cells to the marginal zone.J Immunol. 2005 Feb 1;174(3):1438-45. doi: 10.4049/jimmunol.174.3.1438. J Immunol. 2005. PMID: 15661902
-
B-cell intrinsic TLR7 signals promote depletion of the marginal zone in a murine model of Wiskott-Aldrich syndrome.Eur J Immunol. 2015 Oct;45(10):2773-9. doi: 10.1002/eji.201545644. Epub 2015 Aug 31. Eur J Immunol. 2015. PMID: 26256668 Free PMC article.
-
TLR activation excludes circulating naive CD8+ T cells from gut-associated lymphoid organs in mice.J Immunol. 2013 May 15;190(10):5313-20. doi: 10.4049/jimmunol.1202280. Epub 2013 Apr 15. J Immunol. 2013. PMID: 23589622
-
Fas receptor signaling is requisite for B cell differentiation.J Leukoc Biol. 2005 Nov;78(5):1106-17. doi: 10.1189/jlb.0105047. J Leukoc Biol. 2005. PMID: 16266974
Cited by
-
Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes.Nat Rev Immunol. 2013 Feb;13(2):118-32. doi: 10.1038/nri3383. Nat Rev Immunol. 2013. PMID: 23348416 Free PMC article. Review.
-
Differential impact of Toll-like receptor signaling on distinct B cell subpopulations.Front Biosci (Landmark Ed). 2012 Jan 1;17(4):1499-516. doi: 10.2741/4000. Front Biosci (Landmark Ed). 2012. PMID: 22201817 Free PMC article. Review.
-
Regulatory B cells in autoimmune diseases and mucosal immune homeostasis.Autoimmunity. 2011 Feb;44(1):58-68. doi: 10.3109/08916931003782189. Epub 2010 Aug 11. Autoimmunity. 2011. PMID: 20701454 Free PMC article.
-
Microbiota and the Response to Vaccines Against Respiratory Virus.Front Immunol. 2022 May 6;13:889945. doi: 10.3389/fimmu.2022.889945. eCollection 2022. Front Immunol. 2022. PMID: 35603203 Free PMC article. Review.
-
Toll-like receptors--sentries in the B-cell response.Immunology. 2009 Nov;128(3):311-23. doi: 10.1111/j.1365-2567.2009.03173.x. Immunology. 2009. PMID: 20067531 Free PMC article. Review.
References
-
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu.Rev.Microbiol. 1977;31:107–133. - PubMed
-
- Conway PL. Microbial ecology of the human large intestine. In: Gibson GR, editor. Human colonic bacteria: role in nutrition, physiology, and pathology. Boca Raton: CRC Press; 1995. pp. 1–24.
-
- Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. - PubMed
-
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI52031/AI/NIAID NIH HHS/United States
- CA016042/CA/NCI NIH HHS/United States
- DK69434/DK/NIDDK NIH HHS/United States
- R01 HD037091/HD/NICHD NIH HHS/United States
- DK46763/DK/NIDDK NIH HHS/United States
- R01 DK069434-04/DK/NIDDK NIH HHS/United States
- P01 DK046763-070002/DK/NIDDK NIH HHS/United States
- P01 DK046763/DK/NIDDK NIH HHS/United States
- HD37091/HD/NICHD NIH HHS/United States
- P30 DK349870/DK/NIDDK NIH HHS/United States
- T32 AI052031/AI/NIAID NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P40 RR018603/RR/NCRR NIH HHS/United States
- R01 DK069434/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

