Alteration of blood-brain barrier integrity by retroviral infection
- PMID: 19008946
- PMCID: PMC2575404
- DOI: 10.1371/journal.ppat.1000205
Alteration of blood-brain barrier integrity by retroviral infection
Abstract
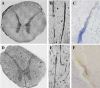
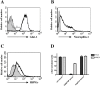
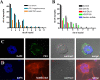
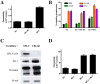
The blood-brain barrier (BBB), which forms the interface between the blood and the cerebral parenchyma, has been shown to be disrupted during retroviral-associated neuromyelopathies. Human T Lymphotropic Virus (HTLV-1) Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) is a slowly progressive neurodegenerative disease associated with BBB breakdown. The BBB is composed of three cell types: endothelial cells, pericytes and astrocytes. Although astrocytes have been shown to be infected by HTLV-1, until now, little was known about the susceptibility of BBB endothelial cells to HTLV-1 infection and the impact of such an infection on BBB function. We first demonstrated that human cerebral endothelial cells express the receptors for HTLV-1 (GLUT-1, Neuropilin-1 and heparan sulfate proteoglycans), both in vitro, in a human cerebral endothelial cell line, and ex vivo, on spinal cord autopsy sections from HAM/TSP and non-infected control cases. In situ hybridization revealed HTLV-1 transcripts associated with the vasculature in HAM/TSP. We were able to confirm that the endothelial cells could be productively infected in vitro by HTLV-1 and that blocking of either HSPGs, Neuropilin 1 or Glut1 inhibits this process. The expression of the tight-junction proteins within the HTLV-1 infected endothelial cells was altered. These cells were no longer able to form a functional barrier, since BBB permeability and lymphocyte passage through the monolayer of endothelial cells were increased. This work constitutes the first report of susceptibility of human cerebral endothelial cells to HTLV-1 infection, with implications for HTLV-1 passage through the BBB and subsequent deregulation of the central nervous system homeostasis. We propose that the susceptibility of cerebral endothelial cells to retroviral infection and subsequent BBB dysfunction is an important aspect of HAM/TSP pathogenesis and should be considered in the design of future therapeutics strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization.J Immunol. 2007 Aug 15;179(4):2576-83. doi: 10.4049/jimmunol.179.4.2576. J Immunol. 2007. PMID: 17675520
-
Human T-Lymphotropic Virus Type 1-Induced Overexpression of Activated Leukocyte Cell Adhesion Molecule (ALCAM) Facilitates Trafficking of Infected Lymphocytes through the Blood-Brain Barrier.J Virol. 2016 Jul 27;90(16):7303-7312. doi: 10.1128/JVI.00539-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252538 Free PMC article.
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
-
Pentosan Polysulfate Demonstrates Anti-human T-Cell Leukemia Virus Type 1 Activities In Vitro and In Vivo.J Virol. 2019 Jul 30;93(16):e00413-19. doi: 10.1128/JVI.00413-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31167921 Free PMC article.
-
Therapeutic strategies in HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP).Cent Nerv Syst Agents Med Chem. 2009 Jun;9(2):137-49. doi: 10.2174/187152409788452090. Cent Nerv Syst Agents Med Chem. 2009. PMID: 20021347 Review.
Cited by
-
Immune activation of human brain microvascular endothelial cells inhibits HIV replication in macrophages.Blood. 2013 Apr 11;121(15):2934-42. doi: 10.1182/blood-2012-08-450353. Epub 2013 Feb 11. Blood. 2013. PMID: 23401273 Free PMC article.
-
Impaired VEGF-A-Mediated Neurovascular Crosstalk Induced by SARS-CoV-2 Spike Protein: A Potential Hypothesis Explaining Long COVID-19 Symptoms and COVID-19 Vaccine Side Effects?Microorganisms. 2022 Dec 12;10(12):2452. doi: 10.3390/microorganisms10122452. Microorganisms. 2022. PMID: 36557705 Free PMC article. Review.
-
High proviral load of human T cell lymphotropic virus type-1 facilitates coronary artery diseases.Iran J Basic Med Sci. 2020 Apr;23(4):500-506. doi: 10.22038/ijbms.2020.36317.8649. Iran J Basic Med Sci. 2020. PMID: 32489565 Free PMC article.
-
Mother-to-Child Transmission of HTLV-1 Epidemiological Aspects, Mechanisms and Determinants of Mother-to-Child Transmission.Viruses. 2016 Feb 3;8(2):40. doi: 10.3390/v8020040. Viruses. 2016. PMID: 26848683 Free PMC article. Review.
-
Innate Immune Sensing of Viruses and Its Consequences for the Central Nervous System.Viruses. 2021 Jan 23;13(2):170. doi: 10.3390/v13020170. Viruses. 2021. PMID: 33498715 Free PMC article. Review.
References
-
- Toborek M, Lee YW, Flora G, Pu H, Andras IE, et al. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25:181–199. - PubMed
-
- Gessain A, Barin F, Vernant JC, Gout O, Maurs L, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. - PubMed
-
- Ozden S, Seilhean D, Gessain A, Hauw JJ, Gout O. Severe demyelinating myelopathy with low human T cell lymphotropic virus type 1 expression after transfusion in an immunosuppressed patient. Clin Infect Dis. 2002;34:855–860. - PubMed
-
- Akizuki S, Nakazato O, Higuchi Y, Tanabe K, Setoguchi M, et al. Necropsy findings in HTLV-I associated myelopathy. Lancet. 1987;1:156–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

