A generic mechanism of emergence of amyloid protofilaments from disordered oligomeric aggregates
- PMID: 19008938
- PMCID: PMC2572140
- DOI: 10.1371/journal.pcbi.1000222
A generic mechanism of emergence of amyloid protofilaments from disordered oligomeric aggregates
Abstract
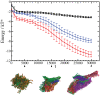
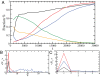
The presence of oligomeric aggregates, which is often observed during the process of amyloid formation, has recently attracted much attention because it has been associated with a range of neurodegenerative conditions including Alzheimer's and Parkinson's diseases. We provide a description of a sequence-indepedent mechanism by which polypeptide chains aggregate by forming metastable oligomeric intermediate states prior to converting into fibrillar structures. Our results illustrate that the formation of ordered arrays of hydrogen bonds drives the formation of beta-sheets within the disordered oligomeric aggregates that form early under the effect of hydrophobic forces. Individual beta-sheets initially form with random orientations and subsequently tend to align into protofilaments as their lengths increase. Our results suggest that amyloid aggregation represents an example of the Ostwald step rule of first-order phase transitions by showing that ordered cross-beta structures emerge preferentially from disordered compact dynamical intermediate assemblies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Self-templated nucleation in peptide and protein aggregation.Phys Rev Lett. 2008 Dec 19;101(25):258101. doi: 10.1103/PhysRevLett.101.258101. Epub 2008 Dec 17. Phys Rev Lett. 2008. PMID: 19113754
-
Interpreting the aggregation kinetics of amyloid peptides.J Mol Biol. 2006 Jul 21;360(4):882-92. doi: 10.1016/j.jmb.2006.05.033. Epub 2006 Jun 5. J Mol Biol. 2006. PMID: 16797587
-
Probing amyloid fibril formation of the NFGAIL peptide by computer simulations.J Chem Phys. 2007 Feb 14;126(6):065101. doi: 10.1063/1.2435358. J Chem Phys. 2007. PMID: 17313247
-
Simulation Studies of Amyloidogenic Polypeptides and Their Aggregates.Chem Rev. 2019 Jun 26;119(12):6956-6993. doi: 10.1021/acs.chemrev.8b00731. Epub 2019 Apr 11. Chem Rev. 2019. PMID: 30973229 Review.
-
Theoretical approaches to protein aggregation.Protein Pept Lett. 2006;13(3):287-93. doi: 10.2174/092986606775338407. Protein Pept Lett. 2006. PMID: 16515457 Review.
Cited by
-
What drives amyloid molecules to assemble into oligomers and fibrils?Biophys J. 2011 Jan 19;100(2):450-8. doi: 10.1016/j.bpj.2010.11.041. Biophys J. 2011. PMID: 21244841 Free PMC article.
-
Amyloid assembly is dominated by misregistered kinetic traps on an unbiased energy landscape.Proc Natl Acad Sci U S A. 2020 May 12;117(19):10322-10328. doi: 10.1073/pnas.1911153117. Epub 2020 Apr 28. Proc Natl Acad Sci U S A. 2020. PMID: 32345723 Free PMC article.
-
Size distribution of amyloid nanofibrils.Biophys J. 2011 Nov 2;101(9):2232-41. doi: 10.1016/j.bpj.2011.09.053. Epub 2011 Nov 1. Biophys J. 2011. PMID: 22067163 Free PMC article.
-
Observation of spatial propagation of amyloid assembly from single nuclei.Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):14746-51. doi: 10.1073/pnas.1105555108. Epub 2011 Aug 26. Proc Natl Acad Sci U S A. 2011. PMID: 21876182 Free PMC article.
-
Unified theoretical description of the kinetics of protein aggregation.Biophys Rev. 2019 Apr;11(2):191-208. doi: 10.1007/s12551-019-00506-5. Epub 2019 Mar 19. Biophys Rev. 2019. PMID: 30888575 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

