Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein
- PMID: 19004960
- PMCID: PMC2612354
- DOI: 10.1128/JVI.01102-08
Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein
Abstract
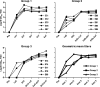
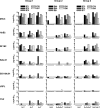
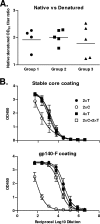
Currently there is limited information about the quality of immune responses elicited by candidate human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env)-based immunogens in primates. Here we describe a comprehensive analysis of neutralizing antibody and T-cell responses obtained in cynomolgus macaques by three selected immunization regimens. We used the previously described YU2-based gp140 protein trimers administered in an adjuvant, preceded by two distinct priming strategies: either alphavirus replicon particles expressing matched gp140 trimers or gp120 core proteins stabilized in the CD4-bound conformation. The rationale for priming with replicon particles was to evaluate the impact of the expression platform on trimer immunogenicity. The stable core proteins were chosen in an attempt to expand selectively lymphocytes recognizing common determinants between the core and trimers to broaden the immune response. The results presented here demonstrate that the platform by which Env trimers were delivered in the priming (either protein or replicon vector) had little impact on the overall immune response. In contrast, priming with stable core proteins followed by a trimer boost strikingly focused the T-cell response on the core sequences of HIV-1 Env. The specificity of the T-cell response was distinctly different from that of the responses obtained in animals immunized with trimers alone and was shown to be mediated by CD4(+) T cells. However, this regimen showed limited or no improvement in the neutralizing antibody responses, suggesting that further immunogen design efforts are required to successfully focus the B-cell response on conserved neutralizing determinants of HIV-1 Env.
Figures


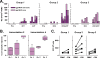
Similar articles
-
Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant.J Virol. 2010 Jun;84(12):5975-85. doi: 10.1128/JVI.02533-09. Epub 2010 Apr 14. J Virol. 2010. PMID: 20392857 Free PMC article.
-
Development of a 3Mut-Apex-Stabilized Envelope Trimer That Expands HIV-1 Neutralization Breadth When Used To Boost Fusion Peptide-Directed Vaccine-Elicited Responses.J Virol. 2020 Jun 16;94(13):e00074-20. doi: 10.1128/JVI.00074-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295908 Free PMC article.
-
Superiority in Rhesus Macaques of Targeting HIV-1 Env gp140 to CD40 versus LOX-1 in Combination with Replication-Competent NYVAC-KC for Induction of Env-Specific Antibody and T Cell Responses.J Virol. 2017 Apr 13;91(9):e01596-16. doi: 10.1128/JVI.01596-16. Print 2017 May 1. J Virol. 2017. PMID: 28202751 Free PMC article.
-
Clade C HIV-1 Envelope Vaccination Regimens Differ in Their Ability To Elicit Antibodies with Moderate Neutralization Breadth against Genetically Diverse Tier 2 HIV-1 Envelope Variants.J Virol. 2019 Mar 21;93(7):e01846-18. doi: 10.1128/JVI.01846-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651354 Free PMC article.
-
Immunogenicity in Rabbits of HIV-1 SOSIP Trimers from Clades A, B, and C, Given Individually, Sequentially, or in Combination.J Virol. 2018 Mar 28;92(8):e01957-17. doi: 10.1128/JVI.01957-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29367243 Free PMC article.
Cited by
-
HIV-1 neutralizing antibodies: understanding nature's pathways.Immunol Rev. 2013 Jul;254(1):225-44. doi: 10.1111/imr.12075. Immunol Rev. 2013. PMID: 23772623 Free PMC article. Review.
-
An HIV-1 Env-Antibody Complex Focuses Antibody Responses to Conserved Neutralizing Epitopes.J Immunol. 2016 Nov 15;197(10):3982-3998. doi: 10.4049/jimmunol.1601134. Epub 2016 Oct 10. J Immunol. 2016. PMID: 27815444 Free PMC article.
-
Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs.J Virol. 2010 Apr;84(7):3270-9. doi: 10.1128/JVI.02252-09. Epub 2010 Jan 6. J Virol. 2010. PMID: 20053749 Free PMC article.
-
Cross-clade HIV-1 neutralizing antibodies induced with V3-scaffold protein immunogens following priming with gp120 DNA.J Virol. 2011 Oct;85(19):9887-98. doi: 10.1128/JVI.05086-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795338 Free PMC article.
-
Neutralizing antibodies to HIV-1 induced by immunization.J Exp Med. 2013 Feb 11;210(2):209-23. doi: 10.1084/jem.20121827. J Exp Med. 2013. PMID: 23401570 Free PMC article. Review.
References
-
- Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 755526-5540. - PMC - PubMed
-
- Barnett, S. W., I. K. Srivastava, E. Kan, F. Zhou, A. Goodsell, A. D. Cristillo, M. G. Ferrai, D. E. Weiss, N. L. Letvin, D. Montefiori, R. Pal, and M. Vajdy. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22339-348. - PubMed
-
- Berglund, P., M. N. Fleeton, C. Smerdou, and P. Liljestrom. 1999. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine 17497-507. - PubMed
-
- Berglund, P., M. Quesada-Rolander, P. Putkonen, G. Biberfeld, R. Thorstensson, and P. Liljestrom. 1997. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res. Hum. Retrovir. 131487-1495. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74627-643. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

