Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells
- PMID: 19003581
- PMCID: PMC2597540
- DOI: 10.1080/01635580802381477
Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells
Abstract
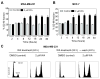
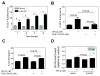
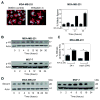
Withaferin A (WA) is derived from the medicinal plant Withania somnifera that has been safely used for centuries in the Indian Ayurvedic medicine for treatment of various ailments. We now demonstrate that WA treatment causes G2 and mitotic arrest in human breast cancer cells. Treatment of MDA-MB-231 (estrogen-independent) and MCF-7 (estrogen-responsive) cell lines with WA resulted in a concentration- and time-dependent increase in G2-M fraction, which correlated with a decrease in levels of cyclin-dependent kinase 1 (Cdk1), cell division cycle 25C (Cdc25C) and/or Cdc25B proteins, leading to accumulation of Tyrosine15 phosphorylated (inactive) Cdk1. Ectopic expression of Cdc25C conferred partial yet significant protection against WA-mediated G2-M phase cell cycle arrest in MDA-MB-231 cells. The WA-treated MDA-MB-231 and MCF-7 cells were also arrested in mitosis as judged by fluorescence microscopy and analysis of Ser10 phosphorylated histone H3. Mitotic arrest resulting from exposure to WA was accompanied by an increase in the protein level of anaphase promoting complex/cyclosome substrate securin. In conclusion, the results of this study suggest that G2-M phase cell cycle arrest may be an important mechanism in antiproliferative effect of WA against human breast cancer cells.
Figures


Similar articles
-
A Comprehensive Review and Perspective on Anticancer Mechanisms of Withaferin A in Breast Cancer.Cancer Prev Res (Phila). 2020 Sep;13(9):721-734. doi: 10.1158/1940-6207.CAPR-20-0259. Epub 2020 Jul 29. Cancer Prev Res (Phila). 2020. PMID: 32727824 Free PMC article. Review.
-
Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo.Cancer Res. 2008 Sep 15;68(18):7661-9. doi: 10.1158/0008-5472.CAN-08-1510. Cancer Res. 2008. PMID: 18794155 Free PMC article.
-
Withaferin a suppresses estrogen receptor-α expression in human breast cancer cells.Mol Carcinog. 2011 Aug;50(8):614-24. doi: 10.1002/mc.20760. Epub 2011 Mar 22. Mol Carcinog. 2011. PMID: 21432907 Free PMC article.
-
Physalis angulata induced G2/M phase arrest in human breast cancer cells.Food Chem Toxicol. 2006 Jul;44(7):974-83. doi: 10.1016/j.fct.2005.11.013. Epub 2006 Jan 19. Food Chem Toxicol. 2006. PMID: 16427178
-
Regulation of Cdc25C activity during the meiotic G2/M transition.Cell Cycle. 2004 Jun;3(6):733-7. Epub 2004 Jun 8. Cell Cycle. 2004. PMID: 15136768 Review.
Cited by
-
Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer.PLoS One. 2012;7(7):e42265. doi: 10.1371/journal.pone.0042265. Epub 2012 Jul 30. PLoS One. 2012. PMID: 22860102 Free PMC article.
-
Withaferin A suppresses tumor promoter 12-O-tetradecanoylphorbol 13-acetate-induced decreases in isocitrate dehydrogenase 1 activity and mitochondrial function in skin epidermal JB6 cells.Cancer Sci. 2013 Feb;104(2):143-8. doi: 10.1111/cas.12051. Epub 2012 Dec 7. Cancer Sci. 2013. PMID: 23107437 Free PMC article.
-
A Comprehensive Review and Perspective on Anticancer Mechanisms of Withaferin A in Breast Cancer.Cancer Prev Res (Phila). 2020 Sep;13(9):721-734. doi: 10.1158/1940-6207.CAPR-20-0259. Epub 2020 Jul 29. Cancer Prev Res (Phila). 2020. PMID: 32727824 Free PMC article. Review.
-
Role of Withaferin A and Its Derivatives in the Management of Alzheimer's Disease: Recent Trends and Future Perspectives.Molecules. 2021 Jun 17;26(12):3696. doi: 10.3390/molecules26123696. Molecules. 2021. PMID: 34204308 Free PMC article. Review.
-
Panepoxydone targets NF-kB and FOXM1 to inhibit proliferation, induce apoptosis and reverse epithelial to mesenchymal transition in breast cancer.PLoS One. 2014 Jun 4;9(6):e98370. doi: 10.1371/journal.pone.0098370. eCollection 2014. PLoS One. 2014. Retraction in: PLoS One. 2023 Dec 29;18(12):e0296553. doi: 10.1371/journal.pone.0296553. PMID: 24896091 Free PMC article. Retracted.
References
-
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. - PubMed
-
- Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883–887. - PubMed
-
- Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67. - PubMed
-
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, et al. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371–1388. - PubMed
-
- Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, et al. First results from the International Breast Cancer Intervention study (IBIS-I): a randomized prevention trial. Lancet. 2002;360:817–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
